Figure 1.
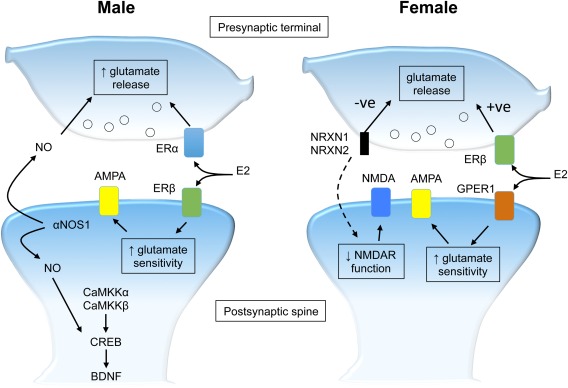
Summary of synaptic plasticity and molecular pathways that differ between the sexes. Neocortical αNOS1 in males has been shown to be involved in in vitro and in vivo synaptic plasticity, with NO acting both pre‐ and postsynaptically. NO and αNOS1 are also more abundant in the male than in the female hippocampus. CaMKKα and CaMKKβ are also more crucial in the male brain for memory tasks, LTP, and CREB transcription. E2 acts via different pre‐ and postsynaptic disposition of oestrogen receptors between the sexes to facilitate increased presynaptic glutamate release and greater postsynaptic glutamate sensitivity; males require presynaptic ERα and postsynaptic ERβ, whereas females employ presynaptic ERβ and postsynaptic GPER1. Deletions of αNrxn1 and αNrxn2 have been shown to impair female behaviors, including anxiety, sociability, and memory, and a loss of presynaptic NRXN2 impairs glutamate release and postsynaptic NMDA receptor function in the neocortex. Dashed line represents the effect of mutations in the NRXN genes that would impair synaptic function. NB: the molecules depicted are expressed in males and females but have specific actions or pre‐/postsynaptic locations only in one or the other as shown.
