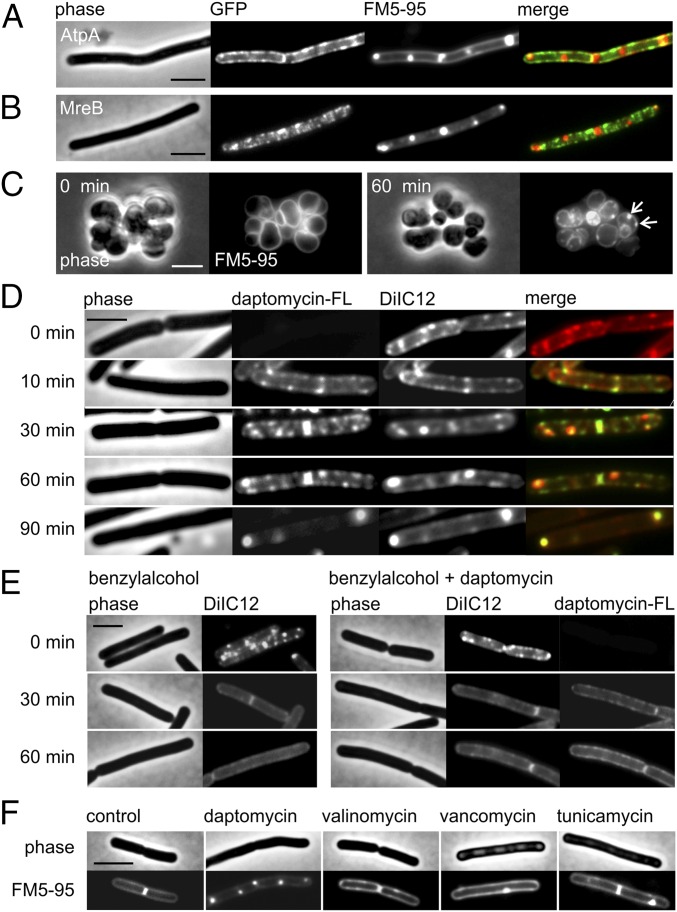
Fig. 4.
Daptomycin does not cause membrane invaginations but clusters fluid lipid domains. (A) Colocalization of daptomycin-induced membrane patches and AtpA–GFP. The membrane was stained with FM5-95. AtpA is distributed uniformly in the cell membrane and is not affected by daptomycin, demonstrating that the strongly fluorescent FM dye foci are not caused by membrane invaginations. (B) Colocalization of daptomycin-induced lipid patches and GFP–MreB. Membrane patches stained with FM5-95 do not overlap with MreB, suggesting that the protein is not involved in the generation of lipid patches. (C) The MreB triple-knockout mutant (ΔmreB Δmbl ΔmreBH) treated with daptomycin and stained with FM5-95. Without the rod shape-determining MreB proteins, the organization of lateral cell wall synthesis is disturbed, resulting in round cells. The presence of lipid patches (arrows) demonstrates independence from the RIF-organizing activity of MreB. (D) B. subtilis 168 costained with the fluorescent RIF dye DiIC12 and fluorescently labeled daptomycin-BODIPY (3 µg/mL). Daptomycin (1 µg/mL) causes native RIFs to deteriorate and fuse into large domains that overlap with daptomycin-BODIPY. (E) The importance of RIFs for daptomycin activity. (Left) The membrane fluidizer benzyl alcohol diminishes RIFs in the B. subtilis membrane. (Right) Pretreatment with benzyl alcohol (10 min) reduces daptomycin clustering in the membrane and prevents the accumulation of fluid lipids into large daptomycin-fluid lipid domains. (F) Lipid patches stained with FM5-95 are characteristic for daptomycin and do not occur after treatment with antibiotics that dissipate the membrane potential (valinomycin) or inhibit lipid II synthesis (vancomycin and tunicamycin). Unless otherwise stated, B. subtilis strains were treated with 2 µg/mL daptomycin for 60 min. (Scale bars: 2 µm.)