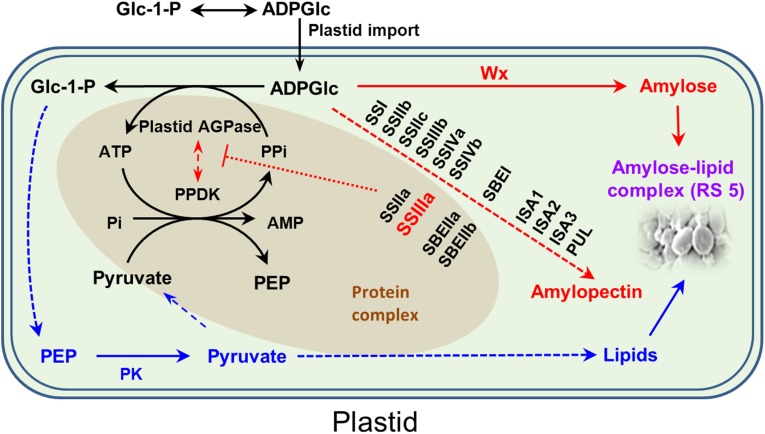
Fig. 6.
A proposed RS biosynthetic pathway in the plastid. Biosynthesis of ADPGlc is brought about primarily by cytosolic AGPase, and ADPGlc is then imported into the plastid for starch biosynthesis. Several amyloplast enzymes exist in a large protein complex, which includes AGPase, PPDK, SSIIa, SSIIIa, SBEIIa, and SBEIIb, and this complex is thought to provide a means to control the partitioning of carbon between starch and lipids. The amyloplast contains a high level of pyrophosphatase, which keeps the concentration of PPi in the stroma very low. The presence of PPDK in a complex with AGPase may enable PPi to be channeled directly to AGPase for the conversion of ADPGlc to G-1-P and subsequently to lipid. The sequestering of PPDK in a protein complex may also prevent a futile cycle operating between PPDK and pyruvate kinase (PK). The starch biosynthetic enzymes in the complex are proposed to inhibit the activity of PPDK and AGPase (red dotted bar). In the absence of a functional SSIIIa protein, the complex is disrupted, and the influence on PPDK and AGPase is reduced, such that more carbon is directed from ADPGlc to Glc-1-P and into lipid. At the same time, the absence of SSIIIa means that relatively more ADPGlc can also be consumed by the Wx protein in the biosynthesis of amylose. This process leads to an increase in amount of amylose–lipid complex and hence RS 5. Dashed arrows indicate multiple steps. AGPase, ADP glucose pyrophosphorylase; ISA, isoamylase; PEP; phosphoenolpyruvate; PUL, pullulanase.