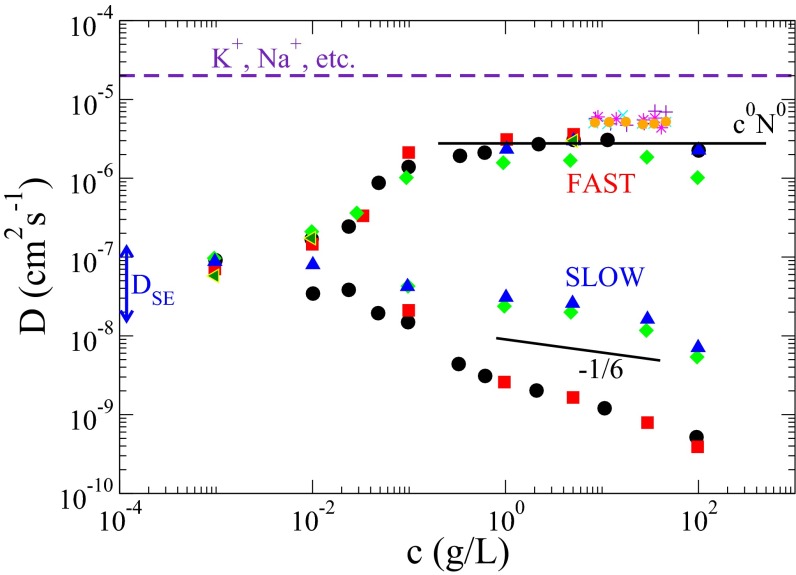
Fig. 1.
Collected data on diffusion coefficient for the fast (ordinary) mode, and the slow (extraordinary) mode, The fast and slow modes emerge for polymer concentrations above a threshold value. For the fully developed two-mode regime, is independent of polymer concentration c and degree of polymerization N over several orders of magnitude and is only about a factor of 4 smaller than the diffusion coefficient of a metallic ion such as Na+ in water. is smaller than by three orders of magnitude and it depends on c and N, suggesting formation of aggregates by similarly charged polymers. Upon addition of electrolytes, the slow mode disappears and progressively becomes smaller, approaching eventually the diffusion coefficient expected from the Stokes–Einstein law. Blue triangle, green diamond, red square, black circle, and green triangle data are from Förster et al. (11) for quarternized poly(2-vinylpyridine) with molecular weight and g/mol and degree of quarternization and 1.0, respectively. Purple plus, aqua cross, purple star, and gold circle data are from Sedlak and Amis (15) for sodium poly(styrene sulfonate) with and g/mol, respectively. The solid lines represent predictions from the present theory.