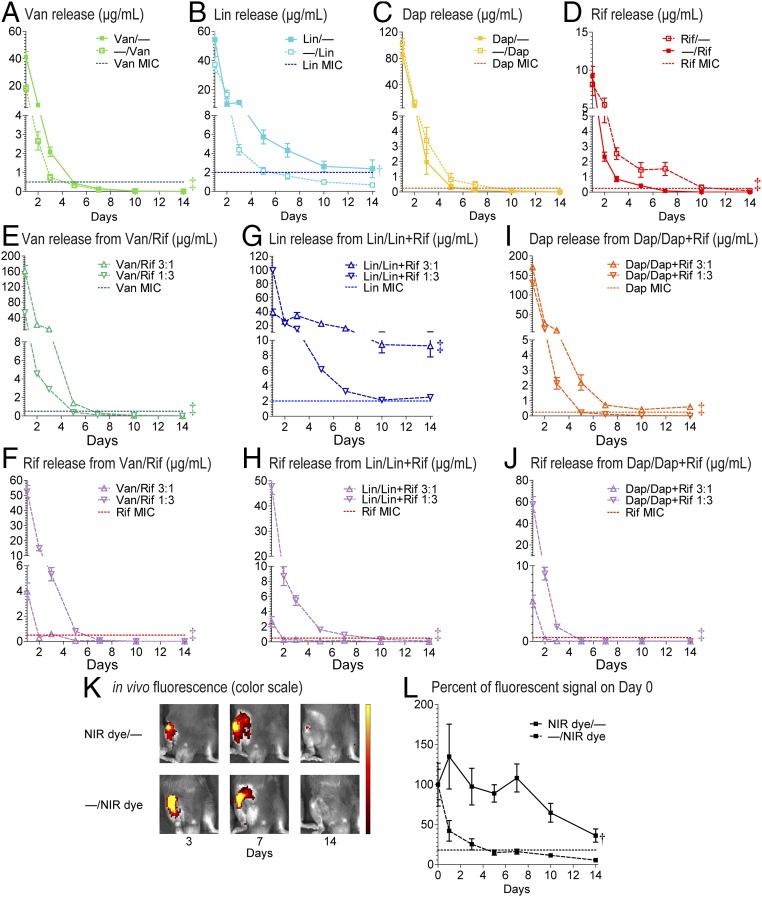
Fig. 3.
Tunability of the composite coatings. In vitro antibiotic release profiles (mean micrograms per milliliter ± SEM) of the antibiotic composite coatings (antibiotics loaded into PLGA and PCL are denoted as shown in Fig. 1E) were measured after varying polymer loading (A–D) and polymer weight ratios (E–J) during electrospinning by placing the coated implants into a new solution of PBS (200 µL) at 37 °C each day for 14 d (n = 10 coated implants per group). Horizontal dotted lines show MIC of Xen36 for each antibiotic: Van (0.5 µg/mL), Lin (2 µg/mL), Dap (0.25 µg/mL), and Rif (0.5 µg/mL). *P < 0.05, †P < 0.01, ‡P < 0.001 for combination antibiotic-loaded coatings vs. single antibiotic coatings (two-way ANOVA) on data from all days (1–14), except D and G, which included data from days 2–14. In vivo release of a NIR fluorescent dye (VivoTag-S 680) loaded into PLGA (NIR dye/−) or PCL (NIR dye/−) was examined in an orthopedic implant mouse model. (K) Representative in vivo fluorescence images. (L) Mean percentage of total radiant efficiency ([p/s]/[µW/cm2]) signal on day 0 ± SEM †P < 0.01 for NIR dye/− vs. −/NIR dye (two-way ANOVA).