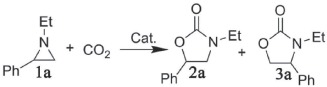
Table 1.
Cycloaddition reaction of CO2 with 1‐ethyl‐2‐phenylaziridine under various conditionsa)
| ||||
|---|---|---|---|---|
| Entry | Catalyst 1 [mg] | T [°C] | Yieldb) [%] | Regio‐selc) |
| 1 | 80 | 25 | 30 | 97:3 |
| 2 | 80 | 80 | 91 | 97:3 |
| 3 | 80 | 100 | >99 | 98:2 |
| 4 | 80 | 120 | 85 | 97:3 |
| 5d) | 80 | 100 | 92 | 98:2 |
| 6 | 40 | 100 | 89 | 98:2 |
| 7e) | 0 | 100 | 45 | 92:8 |
| 8f) | 0 | 100 | 43 | 92:8 |
| 9g) | 0 | 100 | 47 | >99 |
| 10 | 0 | 100 | 79 | 98:2 |
| 11h) | 40 | 100 | 95 | 96:4 |
a)Reaction conditions: 1‐ethyl‐2‐phenylaziridine (294.4 mg, 2.0 mmol), solvent‐free, catalyst 1, TBAB (32.4 mg, 0.1 mmol), CO2 (2.0 MPa), 12 h, 80 mg catalyst 1 loading (based on metal center, about 10 mol%);
b)Total yield of 2a and 3a determined by 1H NMR using 1,3,5‐trimethoxybenzene as an internal standard;
c)Molar ratio of 2a to 3a;
d)CO2 (1.0 MPa);
e)TBAB (32.4 mg, 0.1 mmol) and Cu(OAc)2 (39.9 mg, 0.2 mmol);
f)TBAB (32.4 mg, 0.1 mmol) and Cu(NO3)2 (37.4 mg, 0.2 mmol);
g)TBAB (32.4 mg, 0.1 mmol) and CuSO4 (31.9 mg, 0.2 mmol);
h)HKUST replaces compound 1 to conduct this reaction, catalyst loading (based on metal center, about 10 mol%).
