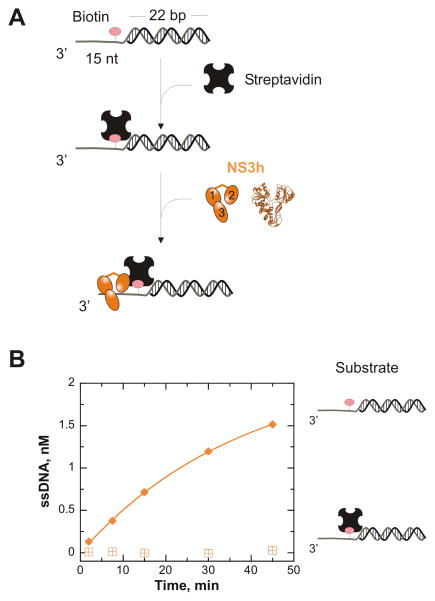
Figure 3.
ATP-independent unwinding by NS3h is halted by the presence of a protein block that prevents sliding into the duplex. A. The streptavidin block was created by incubating 2 nM DNA substrate containing a biotin-thymidine analogue (pink) at position 12 with 120 nM streptavidin (black tetramer) at 37°C prior to initiating the ATP-independent unwinding reaction. The streptavidin-blocked DNA substrate was mixed with 500 nM NS3h (orange) to initiate the ATP-independent unwinding reaction. B. The presence of streptavidin (
 ) halted NS3h unwinding of the biotinylated substrate, T15(bio·dT-12)-22 bp. NS3h was able to unwind this substrate in the absence of streptavidin (
) halted NS3h unwinding of the biotinylated substrate, T15(bio·dT-12)-22 bp. NS3h was able to unwind this substrate in the absence of streptavidin (
 ).
).