Figure 7.
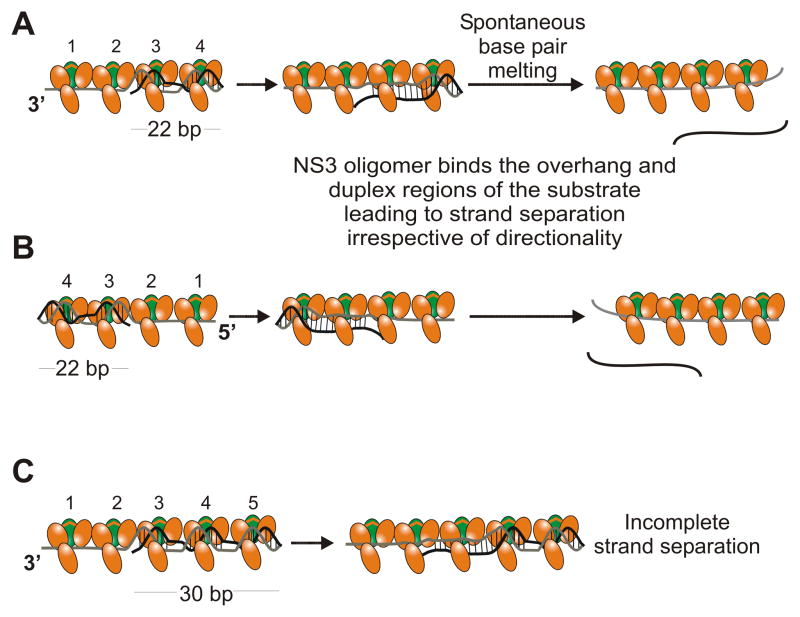
ATP-independent unwinding of DNA substrates is mediated by an oligomeric form of NS3. A. Multiple molecules of NS3 (protease domain shown in green, helicase domain in orange) are capable of binding both the ssDNA tail and duplex regions of a substrate.41 Preferential binding of NS3 to the junction properly aligns the oligomer to optimally interact with the duplex leading to direct separation of base pairs in the duplex. NS3 is capable of disrupting a sufficient number of base pairs such that duplex lengths of 22 bp can separate at 37 °C. B. On a substrate containing a 5′-tail, protein-protein interactions guide NS3 to bind to the duplex region, after initial binding to the ssDNA, which leads to melting of the duplex. C. ATP-independent unwinding of DNA substrates by NS3 oligomer is length-dependent. Slightly longer duplexes are sufficiently stable to remain intact despite binding and melting of some of the dsDNA.