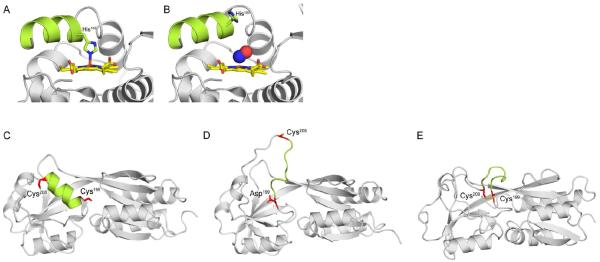
Figure 2. Mechanisms of signal transduction in response to NO· and H2O2.
(A) Reduced H-NOX from Shewanella oneidensis with the penta-coordinated heme (yellow) bound to His103. (B) Binding of NO· (blue and red spheres) to the H-NOX heme severs the bond between His103 and Fe2+. Free His103 rotates the αF helix (green), activating signal transduction. Recent structures of the H2O2 sensor OxyR from Pseudomonas aeruginosa have revealed the molecular mechanism of sensing and signal transduction in response to H2O2. (C) Peroxidatic Cys199 and Cys208 are 15–17Å apart in opposite ends of an α-helix (green). (D) Oxidation of Cys199 by H2O2 produces a sulfenic acid (-SOH) intermediate that is mimicked by a C199D mutation. Oxidation of Cys199 triggers new hydrogen-bonding interactions between the sulfenylated group and Arg201 and Phe200 (not shown). (E) The new hydrogen-bonding disorganizes the intervening α-helix, increasing the reactivity of Cys208 and facilitating formation of a disulfide. (C) and (D) show structures of reduced and OxyR C199D proteins from P. aeruginosa, whereas (E) shows oxidized OxyR from Escherichia coli. PDB codes 4U99, 4U9B, 4Y0M, 4XWS, and 1IBA were used to generate the images in panels (A–E), respectively.