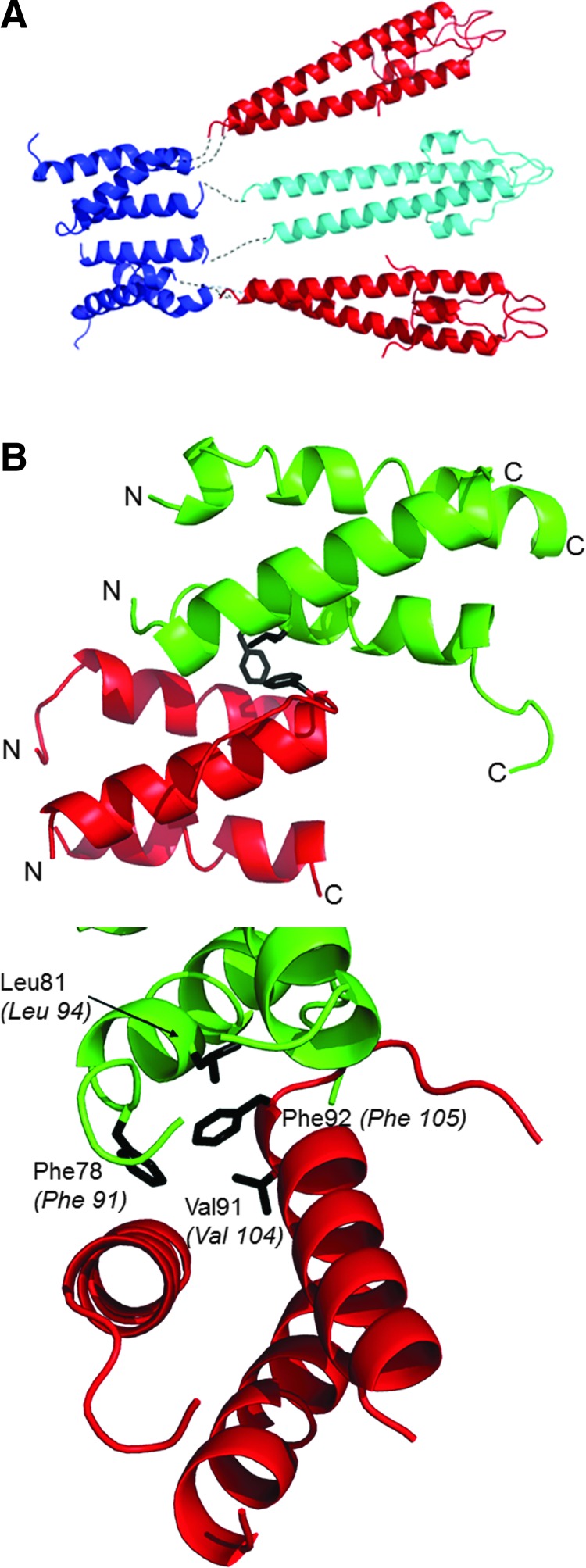
FIG. 4.
Interaction between GpsB domains. (A) A priori hypothetical arrangement of the N- and C-terminal domains of GpsB, as described in the text. N-LmGpsB dimers are colored cyan and red; C-LmGpsB trimers blue. The putative path taken by interdomain linkers is represented as a black dotted line. Note the N-LmGpsB dimer colored cyan is covalently linked to two separate C-LmGpsB trimers and thus forms a bridge between two trimers that would stabilize association into a hexamer. (B) The interface between adjacent (red and green) C-BsGpsB molecules as observed in its crystal lattice, with the key hydrophobic interfacial residues F78, L81, and F92 drawn as sticks and colored black. The lower panel also shows V91, a residue in the hydrophobic core of the C-BsGpsB trimer, which packs against the interfacial residue F92. In the lower panel, the numbering of the Listeria monocytogenes GpsB equivalents of BsGpsB F78, L81, V91, and F92 is shown in italics in parentheses.