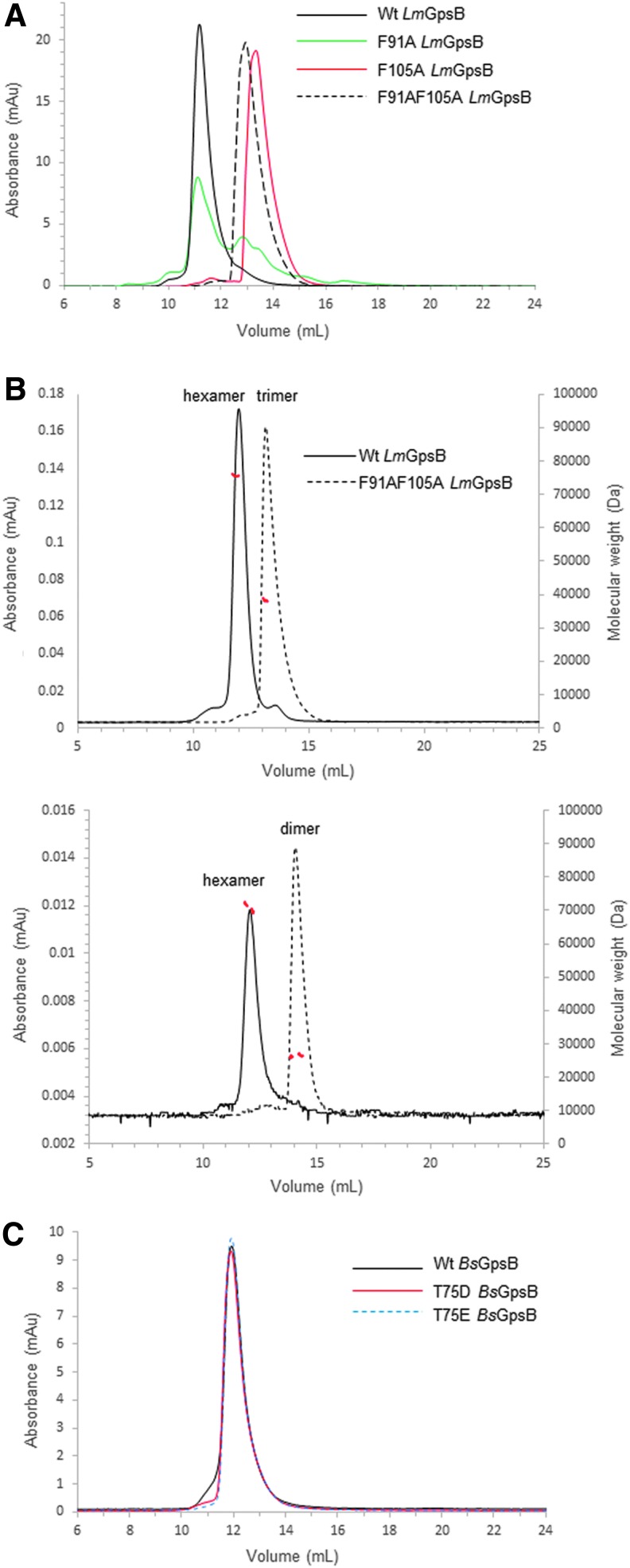
FIG. 5.
SEC and SEC-MALS analysis of GpsB proteins. (A) SEC analysis of wild-type LmGpsB (solid black line), LmGpsBF91A (solid green line), LmGpsBF105A (solid red line), and LmGpsBF91AF105A (dashed black line) proteins at 3 mg/ml concentration. (B) SEC-MALS analysis of wild-type LmGpsB (solid black line) and LmGpsBF91AF105A (dashed black line) proteins. The chromatograms represent analysis at two injected protein concentrations of 8 mg/ml (top) and 0.5 mg/ml (bottom). The deconvoluted molecular masses of the eluting species (red dashed lines) are plotted on the right hand axis. The average of 76 kDa and 38.9 kDa across the major peak for wild-type LmGpsB and LmGpsBF91AF105A at 8 mg/ml is consistent with the theoretical mass of a hexamer (79 kDa) and trimer (38.6 kDa). At lower protein concentrations, the average mass of LmGpsBF91AF105A is 26 kDa, consistent with the theoretical mass of a dimer (25.8 kDa), whereas the wild-type LmGpsB remains hexameric. (C) SEC of wild-type BsGpsB (solid black line), BsGpsBT75E (dashed blue line), and BsGpsBT75D (solid red line) mutants, all at 1 mg/ml. These phosphomimetic mutations do not introduce any significant change to the oligomeric state of BsGpsB. SEC, size exclusion chromatography.