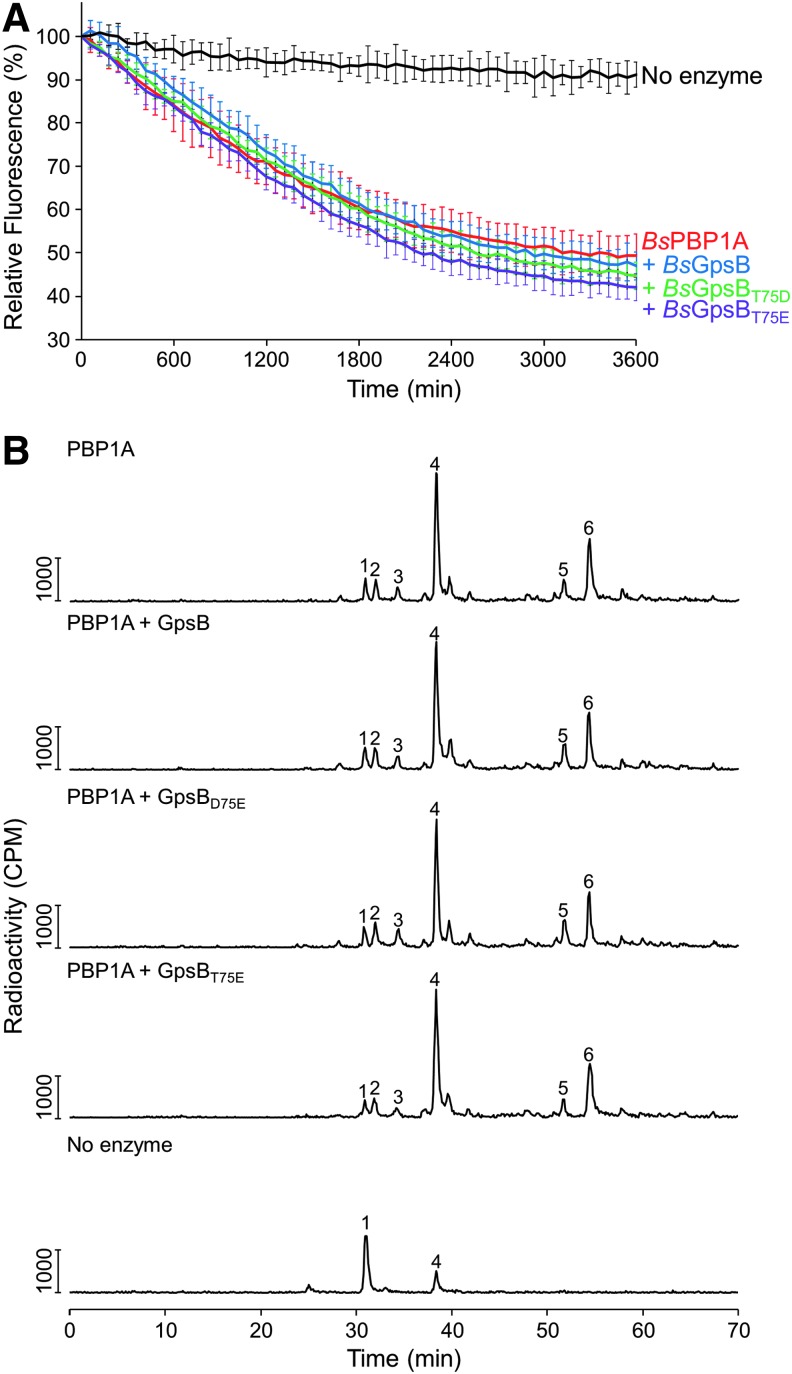
FIG. 8.
GpsB does not affect the enzyme activities of PBP1 in vitro. (A) The GTase activity of BsPBP1 in the presence (blue line) and absence of wild-type BsGpsB (red line), BsGpsBT75D (green line), and BsGpsBT75E (magenta line) was measured using fluorescently labeled lipid II as described previously.40 In the absence of BsPBP1 (black line), no lipid II consumption is observed. Each measurement is shown as the mean ± SD (n = 3). (B) HPLC chromatograms from in vitro PG synthesis assays. BsPBP1 in the presence and absence of BsGpsB was incubated with radiolabeled amidated lipid II-m-DAP. The resultant PG was digested, boiled, and reduced before the resulting muropeptides were separated by HPLC. Peak 1—disaccharide pentapeptide(NH2) monophosphate resulting from unused substrate and glycan chain ends; peak 2—disaccharide tetrapeptide(NH2) derived from GTase and carboxypeptidase activity; peak 3—disaccharide pentapeptide (nonamidated) from the GTase activity on contaminating nonamidated substrate; peak 4—disaccharide pentapeptide(NH2) resulting from the GTase activity; peak 5—bis-disaccharide tetrapeptide(2NH2) resulting from GTase, TPase, and carboxypeptidase activities; peak 6—bis-disaccharide tetrapentapeptide(2NH2) resulting from GTase and TPase activities. HPLC, high-pressure liquid chromatography.