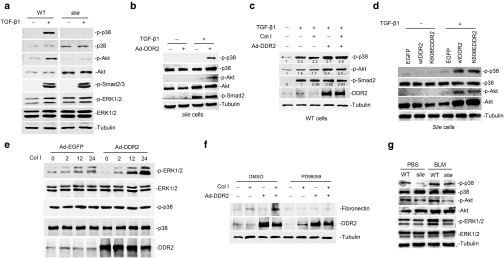
Figure 3.
The protein kinases involved in mediating discoidin domain receptor 2 (DDR2) control of lung myofibroblast activation. (a) DDR2 deficiency in lung fibroblasts impairs the transforming growth factor (TGF)-β1-induced activation of p38 and Akt. Wild-type or slie lung fibroblasts were treated with TGF-β1 for 30 minutes and the cell lysates were then subjected to immunoblot analysis of the indicated proteins. (b,c) The influence of DDR2 overexpression and collagen treatment on TGF-β1 signaling. The indicated lung fibroblasts were infected with recombinant adenovirus for 24 hours and then were treated with either TGF-β1 or the combination of TGF-β1 and collagen I. For the combination treatment, the cells were firstly incubated with or without 10 μg/ml collagen solutions and then stimulated with TGF-β1 for another 30 minutes. (d) DDR2 affects TGF-β1 pathway independent of its activation status. Slie lung fibroblasts were infected lentivirus expressing either wild-type DDR2 or a kinase dead mutant form of DDR2 (K608EDDR2). Fortyeight hours after virus infection, the cells were treated with collagen I for 2 hours and then stimulated with TGF-β1 for another 30 minutes. (e) DDR2 strengthens the collagen I-triggered activation of ERK1/2. Mouse lung fibroblasts expressing EGFP or DDR2 were treated with collagen I (10 μg/ml) for various durations. (f) ERK1/2 cascade is responsible for the collagen I-induced lung myofibroblast activation. Twenty four hours after adenovirus infection, mouse lung fibroblasts were cultured on collagen-coated surface for another 24 hours in the presence of dimethyl sulfoxide or 20 μmol/l PD98059. (g) The kinase activities in bleomycin-injured mouse lung. ERK, extracellular signal-regulated kinase; EGFP, green fluorescent protein.