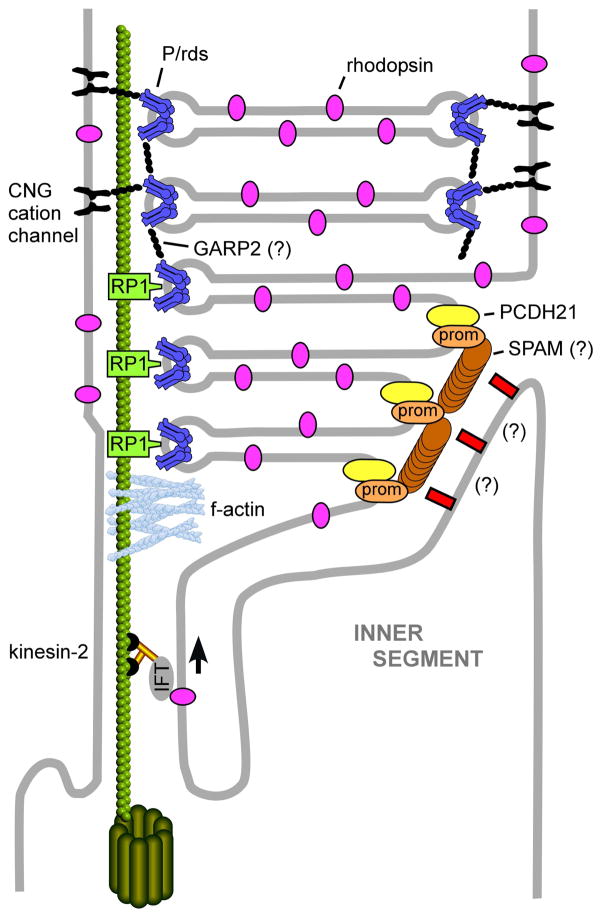
Figure 8. Distributions of OS-resident proteins with likely roles for organelle structure.
Schematic drawing (not to scale) of the basal rod OS shows the locations of the proteins discussed in this article. Kinesin-2 (black/gold) drives anterograde transport of IFT particles and cargo into the OS. Retrograde IFT transport (by dynein) is not illustrated. F-actin (light blue) is localized at the OS base in association with axonemal microtubules. These microfilaments are required for the initiation of new disk evaginations. Rhodopsin (magenta) is present in the ciliary plasma membrane, OS plasma membrane, and OS disk lamellar membranes. Rhodopsin abundance has a dose-dependent effect on disk diameter. Prominin-1 (prom; orange) is present at the edges of basal evaginations, where it interacts with PCDH21 and potentially SPAM. Prominin-1 may function to maintain disk edge membrane curvature. SPAM (brown) localization is putative, and based on its documented interaction with prominin-1 in Drosophila photoreceptors. SPAM function remains to be determined. PCDH21 (yellow) is present at the edges of basal evaginations, where it interacts with prominin-1. The identity (and interactions) of tethers (red rectangles), which link nascent disk edges and the IS plasma membrane remains to be determined. P/rds and P/rds-rom1 complexes (dark blue) are present in disk rim domains. P/rds likely plays a direct role for generating membrane curvature and may participate in disk-disk tethering. Rom-1 modulates P/rds function. RP1 (light green) is associated with axonemal microtubules at the IS-OS junction. RP1 may link nascent OS disks to the axoneme to govern their morphogenesis and stacking. The CNG cation channel (black) is present in the OS plasma membrane. In addition to its primary role for regulating OS permeability, the CNG cation channel contributes to OS structural stability by tethering the plasma membrane to disk rims via interaction with P/rds. GARP-2 (black) is present at OS disk rims. It may contribute to the long-term structural stability of OS disk stacking interactions and/or cGMP phosphodiesterase localization (not shown).