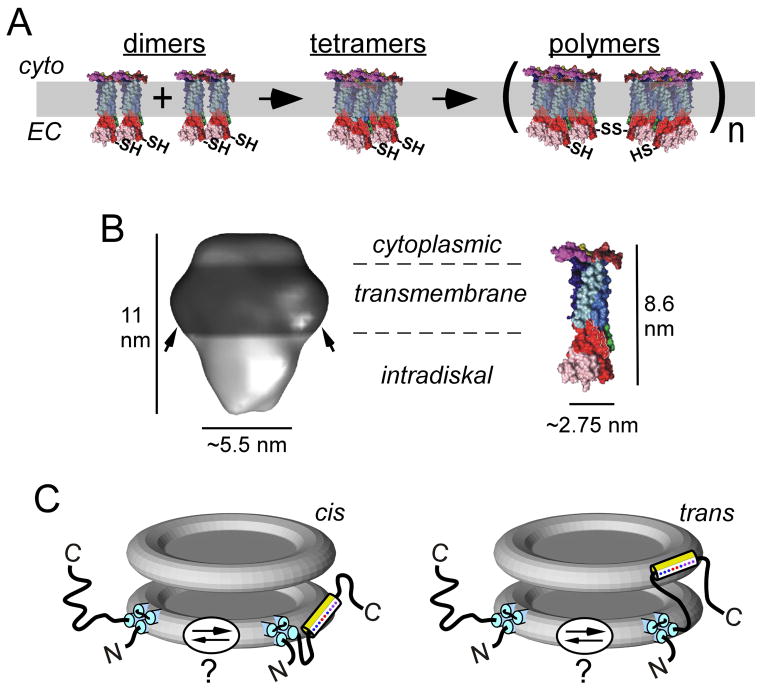
Figure 9. P/rds oligomerization, gross structure, and topology at rod OS disk rims.
A) P/rds dimers (modeled using CD81 monomers, described below) are likely assembled at the biosynthetic level and must undergo additional steps of oligomerization to function in support of OS morphogenesis. Adapted from (Goldberg, 2006). B) Left: 3D structure of the detergent-solubilized P/rds:rom1 heterotetramer. Arrows indicate a presumed annulus of detergent molecules bound at the transmembrane domains. Adapted from (Kevany et al., 2013). Right: 3D model of CD81, a homologous tetraspanin with a mass (26 kDa) somewhat smaller than that of P/rds (39 kDa). Image adapted from (Seigneuret, 2006). The dimensions determined for the P/rds:rom1 heterotetramer are consistent with a complex of four tetraspanin monomers. C) Topology of P/rds in the disk rim, showing hypothesized partitioning of the C-terminal amphipathic helix. The amphipathic helix is leashed to the P/rds transmembrane domain by a disordered region sufficient to bridge adjacent disks, potentially allowing trans insertion.