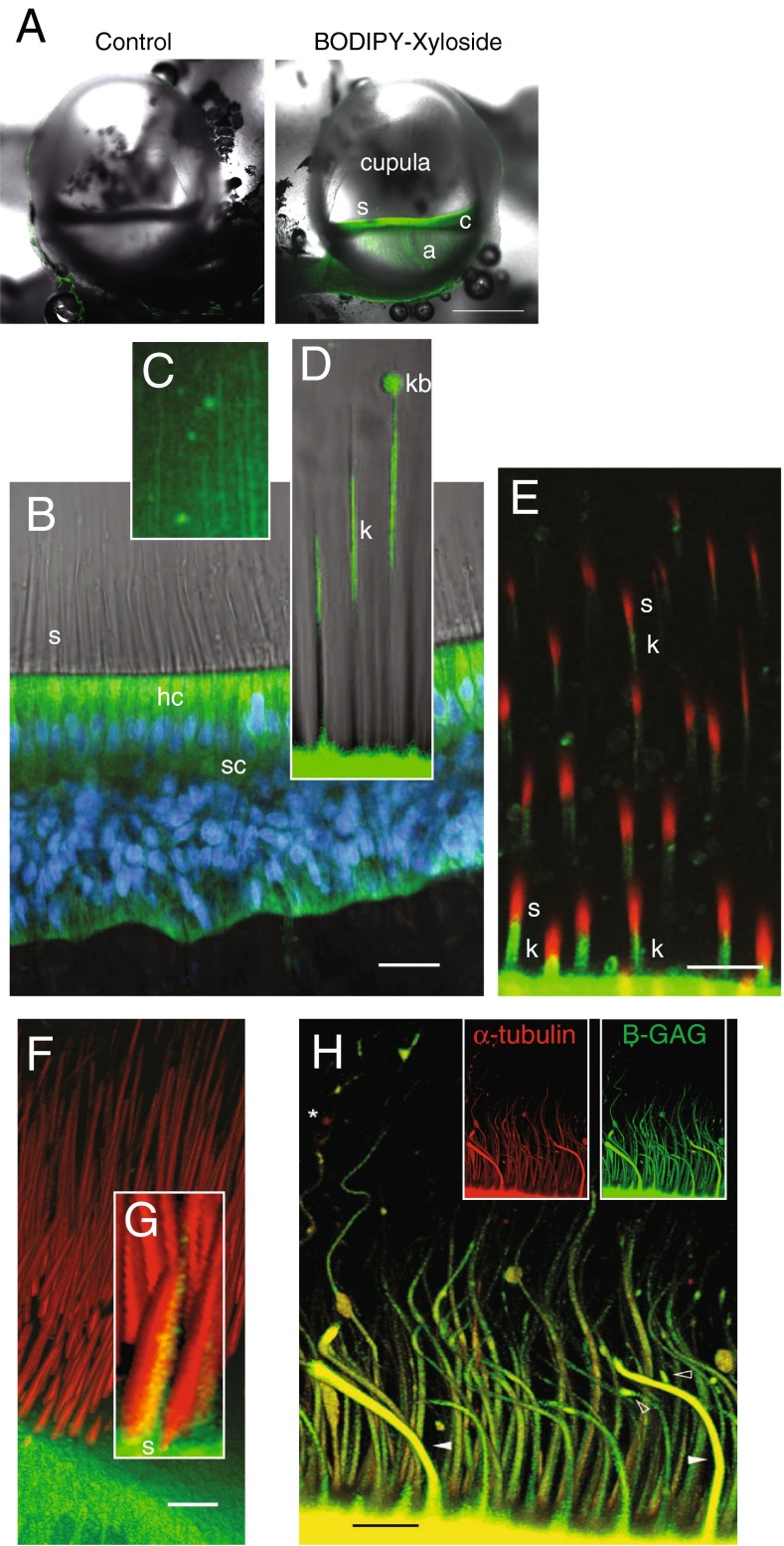
FIG. 2.
In vivo properties of BX fluorescence in the crista ampullaris. A Bilateral confocal images of the left (control, BODIPY-FL alone) and right ampulla (BX) from the same animal, 5 h postadministration of each (scale bar 500 μm). BX-GAGs developed rapidly in hair cells and supporting cells within the sensory epithelium (c) but was slower to appear in the glycocalyx enveloping projections of afferent neurons in the crista (a). BX-GAGs also developed within the cupula and adjacent to stereocilia (s). B Within 15 min post-BX administration; BX-GAGs (green) were visible in hair cells (hc) and supporting cells; nuclei (DAPI, blue) were also visualized for cellular structure and orientation. Stereocilia (s) hair bundles are shown with transmitted light. C Weak lines of BX-GAG fluorescence running from the apical surface of hair cells to the apex of the ampulla developed within the cupula. D BX-GAGs appeared in a glycocalyx structure around kinocilia (k) and in some cases appeared to be terminated by a fluorescent bulb-like structure (kb) located ∼80 μm above the apical surface of hair cells at the tip. Two-photon z-stack sections through hair bundles show stereocilia (phalloidin; s, red) adjacent to BX-GAGs enveloping kinocilium (k; green). The kinocilia (k) appears below stereocilia (s) due to the angle of the optical section through the hair bundles. F 3D rendering from the same z-stack as in E of hair cells and bundles projecting into the cupula. Inset G, BX-GAGs on the kinocilia side of the hair bundle (scale bars 50 μm). H Representative kinocilia (acetylated α-tubulin antibody, red), colocalized with BX-GAGs, in the crista ampullaris, following 4 h post-BX treatment in vivo. BX-GAGs appearing as glycocalyx (white arrows) surrounding kinocilia inside (yellow). Areas along kinocilia appear to have larger diameters due to handling of tissues and bending of the kinocilia that occurred (arrows). Some regions of kinocilia did not appear to have the BX-GAG glycocalyx (star; scale bar: 15 μm)