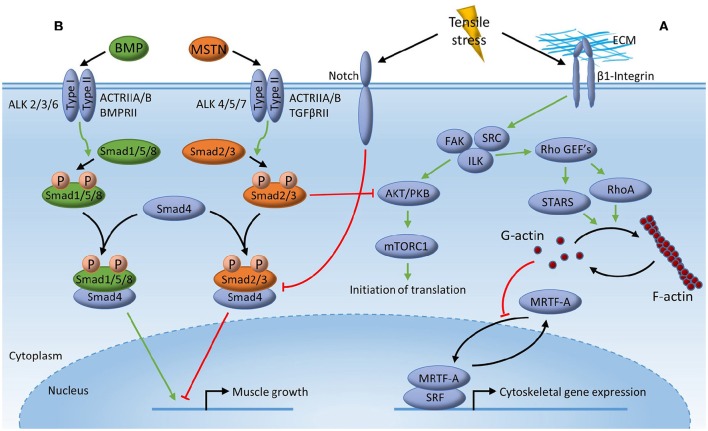
Figure 2.
Mechanotransduction for muscle mRNA transcription. Tensile stress inherent of mechanical deformation may stimulate muscle mRNA transcription through; (A) deformation of membrane-associated β1-Integrin activating focal adhesion kinase (FAK), integrin-linked kinase (ILK) and SRC, which then promotes activation of striated muscle activator of Rho signaling (STARS) and Ras homolog gene family member A (RhoA) through Rho guanine nucleotide exchange factors (GEFs) leading to polymerization of globular actin (G-actin) into filamentous actin (F-actin). Release of cytoplasmic G-actin from myocardin-related transcription factor (MRTF) then allows MRTF to translocate to the nucleus to act as a co-transcription factor with transcription factor serum response factor (SRF), leading to gene expression of multiple muscle myofibrillar and cytoskeletal genes; (B) competitive inhibition of Myostatin (MSTN) signaling by Bone Morphogenetic Protein (BMP) signaling through the common mediator small mother of decapentaplegic 4 (Smad4). Binding of MSTN to its receptor, leads to phosphorylation of Smad2/3 enabling formation of a transcriptional complex with Smad4, which then translocate to the nucleus to modulate transcriptional events resulting in impaired muscle growth. BMP leads to phosphorylation of Smad1/5/8 resulting in the possible formation of a Smad1/5/8-Smad4 transcriptional complex resulting in expression of genes important for muscle growth. Tensile stress inherent of mechanical deformation limits smad2/3 signaling through the membrane-associated protein Notch thereby allowing Smad1/5/8 signaling resulting in muscle accretion.