Figure 1.
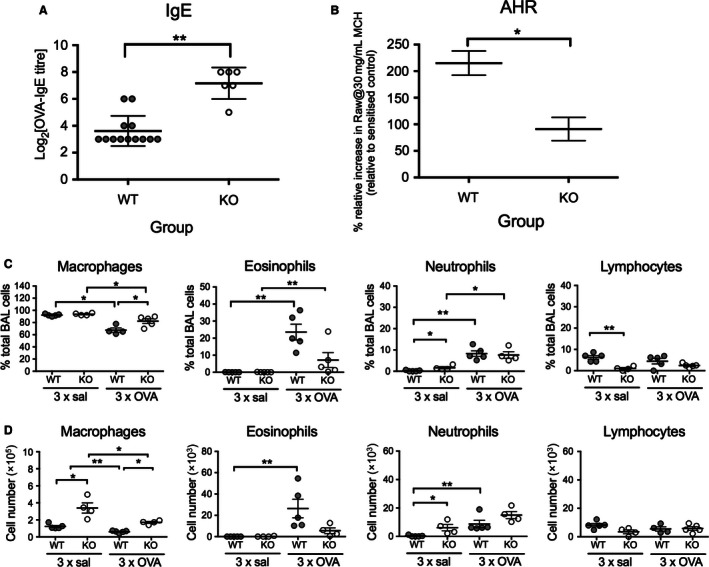
Clinical parameters of OVA‐induced experimental allergic airways disease in CD103−/− and WT BALB/c mice. Adult BALB/c WT and CD103 KO mice were sensitized with OVA in aluminum hydroxide i.p. on days 0 and day 14, then challenged with either OVA or saline aerosols on days 21–23. (A) Sera were collected 24 h after last aerosol and OVA‐specific IgE determined by passive cutaneous anaphylaxis (PCA) as described in Materials and Methods (2 independent experiments, n > 6 mice/group/experiment). (B) Mice received a single OVA or saline aerosol challenge on day 21, 24 h prior to methacholine challenge (MCh) on day 22 and measurement of airways hyper‐responsiveness (AHR) by forced oscillation technique as described in Materials and Methods. Data indicate airways resistance, in response to 30 mg/ml MCh, in WT and CD103 KO mice relative to their respective OVA‐sensitized control. (Two independent experiments, n = 8 to 12 mice/group). (C) and (D) BALF was collected 24 h after final aerosol and the percentages (C) and total numbers (D) of macrophages, eosinophils, neutrophils, and lymphocytes determined by differential staining and counting as described in Materials and Methods (n = 5 mice/group; data are means +/− SEM). *P < 0.05, **P < 0.01 by two‐tailed Mann–Whitney U‐test. AHR, airways hyper‐responsiveness; BLF, Broncheoalveolar lavage fluid; WT, wild type; KO, knockout; OVA, ovalbumin.
