Abstract
Lignocellulose is one of the most abundant renewable feedstocks that has attracted considerable attention as a substrate for biofuel and biochemical production. One such biochemical product, lactic acid, is an important fermentation product because of its great potential for the production of biodegradable and biocompatible polylactic acid. High-titer lactic acid production from lignocellulosic materials has been achieved recently; however, it requires biodetoxification or results in large amounts of waste washing water. In this study, we employed two alkaline pretreatment methods and compared their effects on lactic acid fermentation of pretreated corncob by Bacillus coagulans LA204 using fed-batch simultaneous saccharification and fermentation under non-sterile conditions. The lactic acid titer, yield, and productivity from 16% (w/w) NaOH-pretreated and washed corncob were 122.99 g/L, 0.77 g/g corncob, and 1.37 g/L/h, respectively, and from 16% NH3-H2O2-pretreated and washed corncob were 118.60 g/L, 0.74 g/g corncob, and 1.32 g/L/h, respectively. Importantly, the lactic acid titer, yield, and productivity from 18.4% NH3-H2O2-pretreated and unwashed corncob by using fed-batch simultaneous saccharification and fermentation reached 79.47 g/L, 0.43 g/g corncob, and 1.10 g/L/h, respectively, demonstrating that this method is possible for industrial applications and saves washing water.
Lignocellulose, the most abundant global source of biomass, has been largely unutilized for biofuel and biochemical production. Over 800 million tons of lignocellulose have been produced in China since 2008, with approximately 505.5 million tons of primary biomass being available for further utilization1. Corncob is one of the most important agricultural residues available in high quantities, with 3.2 to 3.6 million tons produced in 2012 in China2. Additionally, corncob possesses great potential value as a raw material for the production of high value added chemicals, fuels, and other industrial products because of its high cellulose and hemicellulose content and high energy density3,4. However, effective utilization of lignocellulosic feedstock is not always practical because of its seasonal availability, scattered location, and prohibitive transportation and storage costs5. Currently, open-field burning of agricultural residues has become the preferred route of disposal; farmers in developing countries, especially in Asia, ignore the potential environmental effects and are unaware of the significance of crop residue returning in the field6. Thus, agro residue burning is widely regarded as a main source of toxic air pollutants, with both short- and long-term effects on human health, and fueling global climate changes7,8.
Lactic acid (LA) is an important biochemical product that has attracted increasing attention because of its widespread application in the food, chemical, cosmetic, and pharmaceutical industries. Furthermore, LA has great potential for the production of biodegradable and biocompatible polylactic acid (PLA) polymers, which has driven the current market development for LA. Fermentative production is the main route for producing LA; the advantages of this method include low production temperature and energy consumption, production of optically pure D- or l-lactic acid (l-LA), and cheap renewable substrates such as lignocellulosic biomass9.
The crystalline structure of lignocellulosic biomass results in two major technical obstacles to LA production: biomass pretreatment and hydrolysis and efficient fermentation of pentose from lignocellulosic hydrolysates. The pretreatments applied to agro residues include physical (size reduction), physicochemical (liquid hot water, steam explosion, and ammonia fiber explosion), chemical (acid, alkaline, alkaline/oxidative, wet oxidation, and ozonolysis), and biological methods10. Acid hydrolysis and steam can be used to hydrolyze hemicellulose into fermentable mono- or oligosaccharides using high temperature or pressure11; and alkaline treatments (lime, sodium hydroxide, wet-oxidation, and soaking with ammonia) provide efficient delignification, resulting in solid residues of cellulose fibers and certain hemicelluloses12,13,14,15,16. A method combining sodium hydroxide (NaOH) pre-extraction and alkaline hydrogen peroxide (H2O2) post-treatment was investigated using corn stover as the substrate. It was found that NaOH first solubilized and removed the easily-extracted lignin and xylan and the oxidizing post-treatment then removed the more recalcitrant lignin from the cell walls17. This combined approach achieved high enzymatic sugar yields from pretreated corn stover using low oxidant loading.
However, pretreatments generate inhibitors (phenolic compounds and formic acid in alkaline-pretreated biomass and hydroxymethyl furfural [HMF] and furfural in acid-pretreated biomass) that repress LA fermentation. Thus, efficient LA production from pretreated biomass requires the removal of these inhibitors prior to fermentation or the use of inhibitor-tolerant bacteria. Moreover, calcium carbonate (CaCO3) or NaOH are required to maintain the neutral or mildly acidic conditions favorable for LA fermentation. The resulting accumulation of sodium lactate or calcium lactate in the fermentation broth can have various stress effects on lactic acid bacteria18, however, like in other fermentation systems, removal of toxic products would improve fermentation yields19. Recently, several lactic acid bacteria, including Lactobacillus strains and Bacillus coagulans, have been reported to produce high-titer LA from lignocellulosic materials. B. coagulans strains, possessing robust inhibitor tolerance, were shown to be suitable for lignocellulosic LA production and were engineered for ethanol production because of their thermophilic growth characteristics and strong pentose homofermentative activity16,20. The LA yield and titer obtained with B. coagulans DSM 2314 reached 0.26 g/g lime-pretreated wheat stover and 40.7 g/L, respectively21. In addition, it has been reported that LA production yield and titer using oil palm empty fruit bunch acid hydrolysate with B. coagulans reached 0.97 g/g and 59.2 g/L, respectively22. The LA yield and titer obtained from acid-pretreated wheat stover via simultaneous saccharification and fermentation (SSF) using B. coagulans IPE22 reached 0.46 g/g acid-pretreated wheat stover and 38.73 g/L, respectively23. Interestingly, the LA yield and titer using B. coagulans LA204 reached 0.68 g/g substrate and 97.6 g/L, respectively, for fed-batch fermentation with 14.4% solid content using NaOH-pretreated and washed corn stover12. However, the major disadvantage of these studies is the substantial volume of waste washing water generated by inhibitor removal. In terms of industrial production, wastewater should be strictly limited because of the high cost of wastewater treatment24. In contrast, significant LA production was obtained from sulfuric acid-pretreated and biodetoxified corn stover by Pediococcus acidilactici DQ2. The LA titer reached 101.9 g/L, however, the yield only reached 0.38 g/g stover and P. acidilactici DQ2 cannot utilize xylose25. A high titer (104.4 g/L) of l-LA was obtained from dilute acid-pretreated and biodetoxified corn stover with <30% solid content using an engineered P. acidilactici TY112 (CGMCC 8664) strain. The yield reached 0.72 g/g glucose from total corn stover without considering xylose unavailability24.
In order to improve lignocellulosic LA production from both cellulose and hemicellulose hydrolysates and reduce the volume of washing water, we compared LA fermentation efficiency using NaOH-pretreated and ammonium-hydrogen peroxide (NH3-H2O2)-pretreated corncob via SSF with strain B. coagulans LA204. The LA yield, titer, and productivity reached 0.43 g/g corncob, 79.47 g/L, and 1.10 g/L/h, respectively, using NH3-H2O2-pretreated and unwashed corncob. This study provides a useful industrial application to avoid the generation of waste washing water.
Results and Discussion
Effect of NaOH and NH3-H2O2 pretreatments on corncob solid composition
Acid and alkaline pretreatments are commonly used to remove lignin from lignocellulosic materials. However, acid pretreatment results in loss of hemicellulose, while alkaline pretreatment maintains most of the hemicelluloses in the solid content and is thus more feasible for biochemical or biofuel fermentation using SSF26. In this study, we selected NaOH and NH3-H2O2 pretreatments to remove the lignin from the corncob and to render the cellulose and hemicellulose accessible to cellulase and hemicellulase. The determined compositional changes in the corncob prior to and post alkaline pretreatment are summarized in Table 1. Following NaOH pretreatment and washing, the cellulosic fraction (as glucose) increased significantly from 37.26% to 59.84%, the hemicellulose fraction (as xylose) decreased from 29.05% to 19.99%, and the lignin content decreased from 19.60% to 6.28%, compared to raw material without pretreatment (Table 1). Subsequent to this pretreatment, 91.71% cellulose, 39.29% hemicellulose, and 18.30% lignin were recovered. These results are in agreement with those of a previous report showing that dilute alkali pretreatment partially solubilizes hemicellulose and leads to swelling as well as disruption of the lignin structure27. The ash content remained constant following NaOH pretreatment and washing. However, subsequent to NH3-H2O2 pretreatment and washing the solid fraction exhibited a 19.54% increase in cellulosic composition and the percentage of hemicellulose, lignin, and ash decreased slightly compared to raw material prior to pretreatment (Table 1). Subsequent to 1-day NH3 pretreatment and 7-days H2O2 pretreatment followed by water washing, 94.43% cellulose, 71.28% hemicellulose, and 68.92% lignin were recovered. While 1-day and 7-days NH3-H2O2 pretreatment resulted in a 4.46% and 13.10% increase in cellulosic fraction, respectively, compared with the raw material, the hemicellulose composition was unchanged; this may be due to the remaining solubilized xylose in the pretreated corncob (Table 1). The lignin content remained the same following the 1-day H2O2 pretreatment but decreased from 19.60% to 16.61% after the 7-day H2O2 pretreatment. The ash content was unaffected by both pretreatments (Table 1). Subsequent to 1-day NH3 pretreatment and 1-day H2O2 pretreatment, 100.02% cellulose, 95.97% hemicellulose, and 93.68% lignin were recovered. After 1-day NH3 pretreatment and 7-days H2O2 pretreatment, 105.03% cellulose, 97.73% hemicellulose, and 82.62% lignin were recovered. These results demonstrate that NaOH pretreatment with subsequent washing can efficiently remove lignin; however, this pretreatment also solubilizes the hemicellulose fraction, resulting in a loss of oligosaccharides. In contrast, the NH3-H2O2 pretreatment preserves both the hemicellulose fraction and the lignin. Although lignin compounds were detected using the Folin-Ciocalteu method in the NH3-H2O2-pretreated corncob, their structures and characteristics may have changed because the presence of lignin compounds did not hinder LA fermentation when NH3-H2O2 pretreated and washed corncob was used. In addition, NH3-H2O2 pretreated and washed corncob had a smaller inhibitory effect than NH3-H2O2 pretreated and unwashed corncob (see below).
Table 1. Composition of the contents in raw and pretreated corncob materials (% dry matter).
| Sample | Cellulose (as glucose,%) | Hemicellulose (as xylose,%) | Lignin (%) | Ash (%) |
|---|---|---|---|---|
| Without pretreatment | 37.26 ± 0.56 | 29.05 ± 0.04 | 19.60 ± 0.64 | 11.17 ± 0.01 |
| NaOH (wash) | 59.84 ± 0.94 | 19.99 ± 0.01 | 6.28 ± 1.05 | 11.34 ± 0.00 |
| NH3-H2O2 (wash, 7d) | 44.54 ± 2.80 | 26.21 ± 0.01 | 17.10 ± 0.15 | 10.16 ± 0.01 |
| NH3-H2O2 (unwash, 1d) | 38.92 ± 0.97 | 28.85 ± 0.01 | 19.00 ± 0.21 | 10.97 ± 0.00 |
| NH3-H2O2 (unwash, 7d) | 42.14 ± 1.15 | 29.12 ± 0.03 | 16.61 ± 0.51 | 10.12 ± 0.00 |
High-titer and high-yield LA fermentation from NaOH-pretreated and washed corncob
In our previous study, B. coagulans LA204 demonstrated remarkably efficient lignocellulosic LA production, with high LA yield and titer and low byproduct generation12. Moreover, LA titer and yield were increased when NaOH-pretreated and washed corncob was used compared with non-pretreated corncob12. In this study, NaOH and NH3-H2O2 were used to pretreat corncob, one of the most abundant agro biomasses in the world, and the LA fermentation ability of B. coagulans LA204 using these materials was compared. The use of 8% NaOH-pretreated and washed corncob as the carbon source, 10 g/L yeast extract as the nitrogen source, and 10 M NaOH solution as the neutralizer resulted in an LA yield of 0.79 g/g total corncob and an LA titer of 62.91 g/L. LA was produced rapidly initially and LA fermentation was nearly complete by 18 h (Fig. 1A); the LA titer reached 56.88 g/L at 18 h and the productivity during this period reached 3.16 g/L/h. Using the same fermentation conditions, with CaCO3 as the neutralizer, the LA yield and titer reached 0.91 g/g total corncob and 72.62 g/L, respectively. During the initial stage of fermentation (0 to 18 h) with CaCO3 as the neutralizer, LA productivity was 2.66 g/L/h, which was slightly lower than that obtained when NaOH was used as the neutralizer; however, LA was continuously produced during the period from 18 to 36 h with a productivity of 1.26 g/L/h (Fig. 1B). These results demonstrate that during the initial stage of fermentation with a lower LA titer, LA was produced more rapidly when NaOH was used as the neutralizer; however, with increasing amounts of LA, soluble sodium lactate had a stronger inhibitory effect on B. coagulans than calcium lactate, which has been previously examined at the transcriptome level18. Finally, a significantly higher amount of LA (0.91 g/g vs. 0.79 g/g corncob) was produced from 8% NaOH-pretreated and washed corncob using CaCO3 as the neutralizer compared to using NaOH as the neutralizer, as demonstrated by independent samples t-test (p < 0.05). Because CaCO3 was sufficient for enhancing LA yield, we performed fed-batch fermentation for high-titer LA production from corncob using CaCO3 as the neutralizer. A similar fermentation curve as shown in Fig. 1B was obtained during the initial stage (0 to 18 h) using 8% NaOH-pretreated and washed corncob and the LA titer reached 45.14 g/L (Fig. 1C). Corncob was then fed to 16% (w/w) and cellulase and yeast extract were fed from 18 to 24 h to maintain the concentrations at 30 filter paper unit (FPU)/g corncob and 10 g/L, respectively. Fermentation was continued for 90 h; the final LA titer and yield were 122.99 g/L and 0.77 g/g, respectively, and the overall productivity was 1.37 g/L/h (Fig. 1C, Table 2).The l-LA optical purity was 98%. These impressive results represent one of the highest levels of LA production from agro biomass reported to date. However, the key issue of efficient inhibitor removal without washing water requires resolution prior to feasible industrial application. Previously, we reported that B. coagulans is sensitive to inhibitors in corn stover created by NaOH pretreatment and that removal of the inhibitors by simple washing enhanced LA yield, titer, and productivity12. However, washing generates a large volume of wastewater that may hinder application; thus, a feasible pretreatment method needs to be developed to avoid the generation of wastewater.
Figure 1. LA fermentation with NaOH-pretreated and washed corncob.
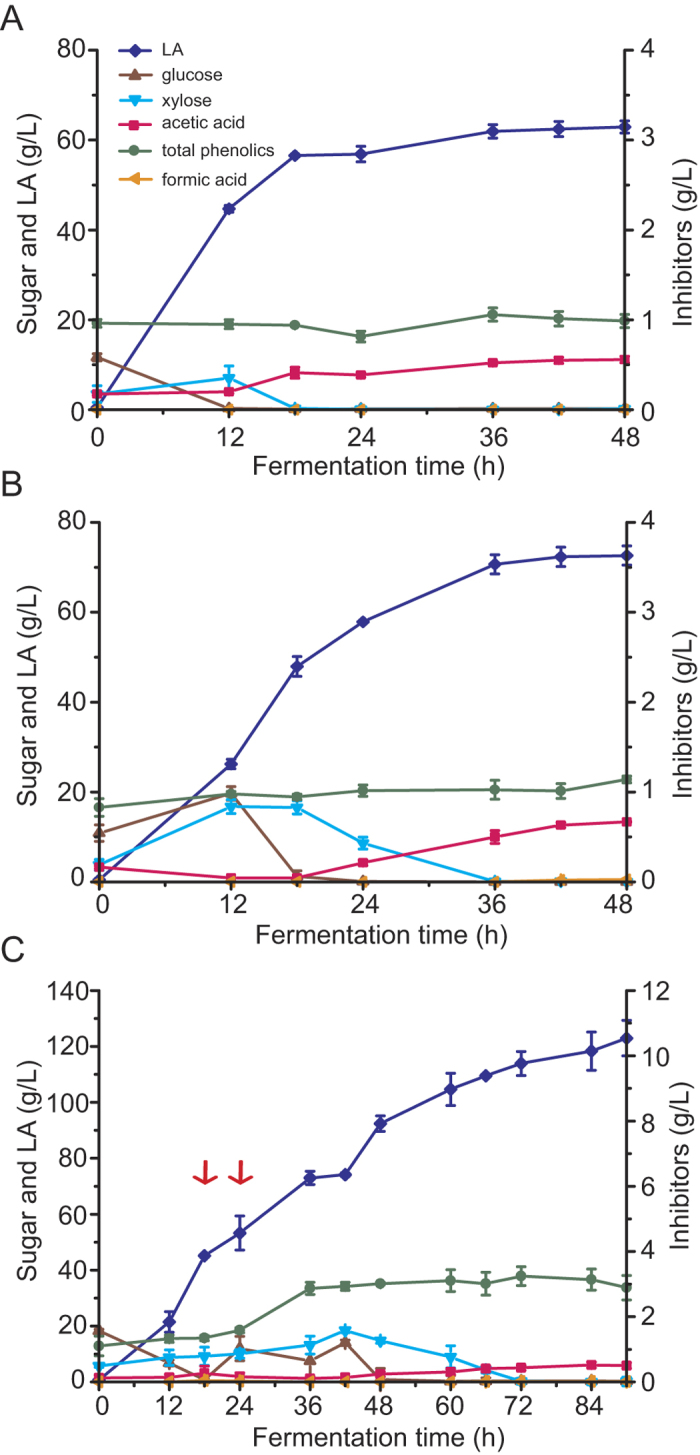
(A) Concentration of sugars (glucose and xylose), LA, and inhibitors (acetic acid, total phenolics, and formic acid) during fermentation of 8% (w/w) NaOH-pretreated and washed corncob at pH6.0 (adjusted by automatic NaOH solution feeding). (B) Fermentation of 8% (w/w) NaOH-pretreated and washed corncob using CaCO3 as a neutralizer. (C) Fermentation of NaOH-pretreated and washed corncob (8% [w/w] initial substrate fed to 16% from 18 h to 24 h) using CaCO3 as aneutralizer. Blue diamond, LA; red square, acetic acid; green circle, total phenolics; brown triangle, glucose; cyan triangle, xylose; orange triangle, formic acid. Experiments were carried out in duplicate and error bars are shown. Where error bars are not visible, they are smaller than the size of the symbol used.
Table 2. Summary of lactic acid fermentation by Bacillus coagulans LA204 using corncob as carbon source by SSF.
| corncob concentration | 8% | 8% | 8–16% | 8% | 8% | 8% | 8–16% | 8–18.4% | 4-8-16% |
|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | NaOH | NaOH | NaOH | NH3-H2O2a | NH3-H2O2b | NH3-H2O2b | NH3-H2O2b | NH3-H2O2b | NH3-H2O2b |
| Washingc | Y | Y | Y-Y | N | N | N | Y-Y | N-N | Y-N-N |
| Neutralizer | NaOH | CaCO3 | CaCO3 | NaOH | NaOH | CaCO3 | CaCO3 | NaOH | NaOH |
| Lactic acid titer (g/L) | 62.91 ± 1.36 | 72.62 ± 2.14 | 122.99 ± 6.37 | 33.67 ± 1.68 | 39.93 ± 2.71 | 42.61 ± 2.35 | 118.60 ± 3.37 | 79.47 ± 3.55 | 84.46 ± 1.21 |
| Lactic acid yield (g/g corncob) | 0.79 | 0.91 | 0.77 | 0.42 | 0.50 | 0.53 | 0.74 | 0.43 | 0.53 |
| Lactic acid productivity (g/L/h) | 1.31 | 1.51 | 1.37 | 0.70 | 0.83 | 0.89 | 1.32 | 1.10 | 1.01 |
| Acetic acid titer (g/L) | 0.56 ± 0.04 | 0.67 ± 0.01 | 0.5 ± 0.09 | 1.06 ± 0.11 | 1.23 ± 0.22 | 1.17 ± 0.16 | 0.45 ± 0.09 | 2.17 ± 0.17 | 1.46 ± 0.07 |
| Acetic acid yield (g/g corncob) | 0.01 | 0.01 | 0.003 | 0.01 | 0.02 | 0.01 | 0.003 | 0.01 | 0.01 |
| Acetic acid productivity (g/L/h) | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 | 0.02 | 0.01 | 0.03 | 0.02 |
aNH3 pretreatment for 1 day and sequential H2O2 pretreatment for 1 day.
b7 days.
cY stands for washed and N for unwashed after alkaline pretreatment.
LA production from NH3-H2O2-pretreated corncob
The LA yield and titer obtained from alkaline-pretreated corn stover via SSF using B. coagulans reached relative high titer and yield12. However, the major disadvantage is the substantial volume of waste washing water generated by inhibitor removal. In the above study, a large volume of water was used to wash the pretreated corn stover in order to remove phenolic inhibitors and to neutralize the pH value. The waste washing water contains large amounts of inhibitors and alkaline, and was hard to be reused. Therefore, one pretreatment method with reduced washing water use need to be developed. NH3 has been shown to remove lignin from lignocellulosic materials14; NH3 residues can be collected by volatilization, eliminating the neutralization step post pretreatment. Furthermore, H2O2 is able to oxidize the phenolic compounds generated by alkaline pretreatment, which may reduce the use of washing water to remove these inhibitors28. Thus, we tested LA fermentation efficiency using NH3-H2O2-pretreated corncob. For the first experiment, LA fermentation was performed using corncob pretreated for 1 day with NH3 and then further treated for 1 day with H2O2 with a NaOH solution as the neutralizer. LA yield, titer, and productivity were 0.42 g/g corncob, 33.70 g/L, and 0.70 g/L/h, respectively (Fig. 2A). For the second fermentation, LA was produced using corncob pretreated for 1 day with NH3 and then further treated for 7 days with H2O2, with a NaOH solution as the neutralizer. The final LA yield, titer, and productivity were 0.50 g/g corncob, 39.93 g/L, and 0.83 g/L/h, respectively (Fig. 2B). LA yield and titer were significantly increased (0.50 g/g corncob vs. 0.42 g/g corncob; and 39.93 g/L vs. 33.67 g/L) when using a substrate with an extended H2O2 pretreatment time compared to 1-day H2O2 pretreatment, as shown by independent samples t-test (p < 0.05). The total phenolic concentration in both experiments was similar; however, we propose that the phenolic compounds from the NH3 pretreatment experiment could be oxidized following extended H2O2 treatment and thus their inhibitory effects were reduced. One significant difference between these fermentations was that a higher amount of LA was produced at 12 h, and importantly, more xylose was liberated using corncob with extended H2O2 pretreatment (Fig. 2A,B). However, when corncob with a short H2O2 pretreatment was used as the substrate, both glucose and xylose were consumed at 12 h and no additional xylose accumulated subsequently (Fig. 2A). These results suggest that the extended H2O2 pretreatment may also enhance xylose liberation from the raw substrate. Because calcium lactate showed less of an inhibitory effect on B. coagulans, we next tested LA fermentation efficiency from NH3-H2O2-pretreated and unwashed corncob using CaCO3 as the neutralizer. LA productivity during the initial stage (0 to 12 h) was lower using CaCO3 as the neutralizer; additionally, more glucose was liberated from the biomass and accumulated in the fermentation culture (Fig. 2C). However, the overall LA titer, yield, and productivity were 42.61 g/L, 0.53 g/g corncob, and 0.89 g/L/h, respectively (Fig. 2C), which was not significantly different compared with the use of NaOH solution as a neutralizer.
Figure 2. LA fermentation of 8% (w/w) NH3-H2O2-pretreated and unwashed corncob.
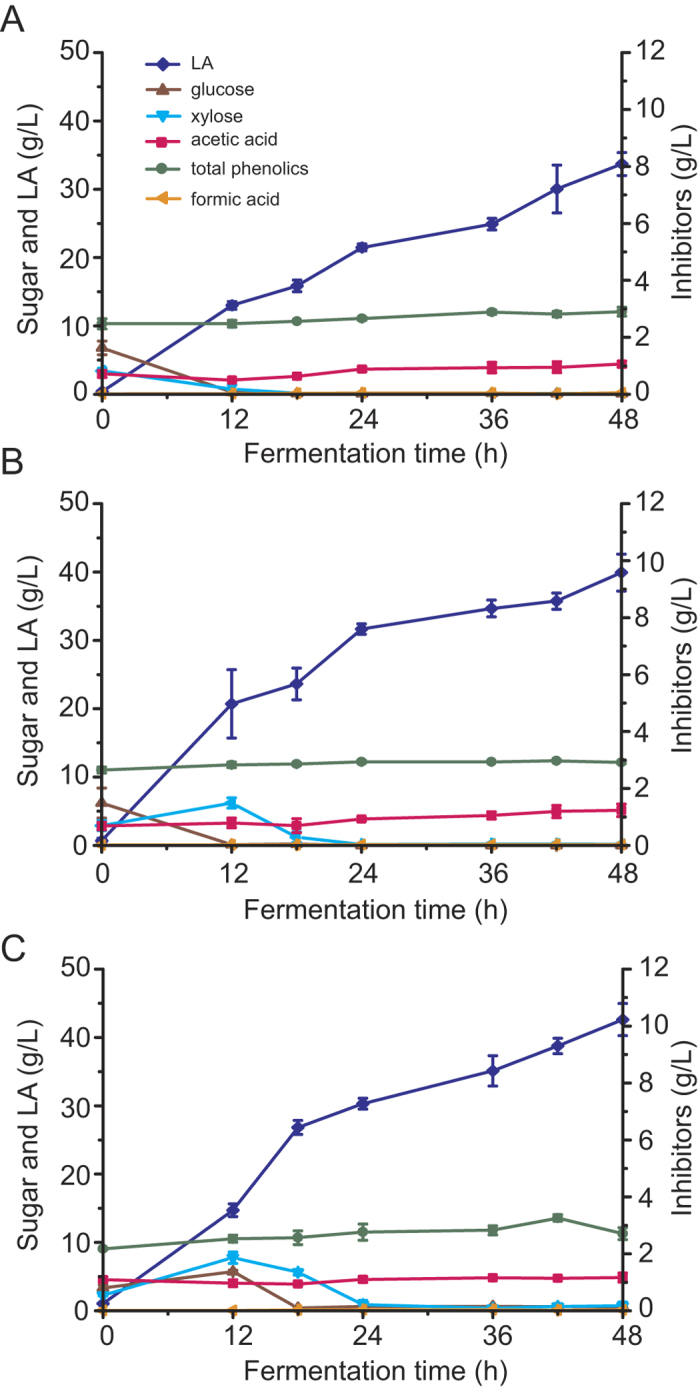
(A) Concentration of sugars (glucose and xylose), LA, and inhibitors (acetic acid, total phenolics, and formic acid) during the fermentation of corncob substrate pretreated for 1 day with NH3 and then treated for 1 day with H2O2 at pH6.0 (adjusted by automatic NaOH solution feeding). (B) Fermentation of corncob substrate pretreated for 1 day with NH3 followed by 7 days of H2O2 treatment at pH6.0 (adjusted by automatic NaOH solution feeding). (C) Fermentation of corncob substrate pretreated for 1 day with NH3 then treated for 7 days with H2O2 using CaCO3 as a neutralizer. Blue diamond, LA; red square, acetic acid; green circle, total phenolics; brown triangle, glucose; cyan triangle, xylose; orange triangle, formic acid. Experiments were carried out in duplicate and error bars are shown. Where error bars are not visible, they are smaller than the size of the symbol used.
LA production in fed-batch fermentation using NH3-H2O2-pretreated corncob as a substrate
In order to compare LA fermentation efficiency using NH3-H2O2- and NaOH-pretreated corncob, we first performed fed-batch fermentation using NH3-H2O2-pretreated and washed corncob. CaCO3 was used as the neutralizer, similar to the fed-batch fermentation experiment using NaOH-pretreated and washed corncob. The results during the initial stage (0 to 18 h) of fermentation using 8% NH3-H2O2-pretreated and washed corncob were similar to those in Fig. 1C; the LA titer reached 43.39 g/L. The corncob was then fed to 16% (w/w) and cellulase was fed to 30 FPU/g corncob from 18 to 24 h. Fermentation was continued for 90 h and the final LA yield and titer reached 0.74 g/g corncob and 118.60 g/L, respectively, and the overall productivity was 1.32 g/L/h (Fig. 3A, Table 2).There was no significant difference in fermentation efficiency compared to when NaOH-pretreated and wash corncob was used, as shown by independent samples t-test. The l-LA optical purity was 98%. These results indicate that NH3-H2O2 pretreatment efficiently promotes sugar liberation by cellulase and hemicellulase for subsequent LA fermentation, although higher levels of lignin were detected in the pretreated corncob (Table 1). However, NH3-H2O2 pretreatment required 8 days to achieve the same LA titer and yield, while the NaOH method required only 3 h for completion.
Figure 3. Fed-batch LA fermentation of corncob with 1-day NH3 pretreatment followed by 7-days H2O2 treatment at pH6.0 (adjusted by automatic NaOH solution feeding).
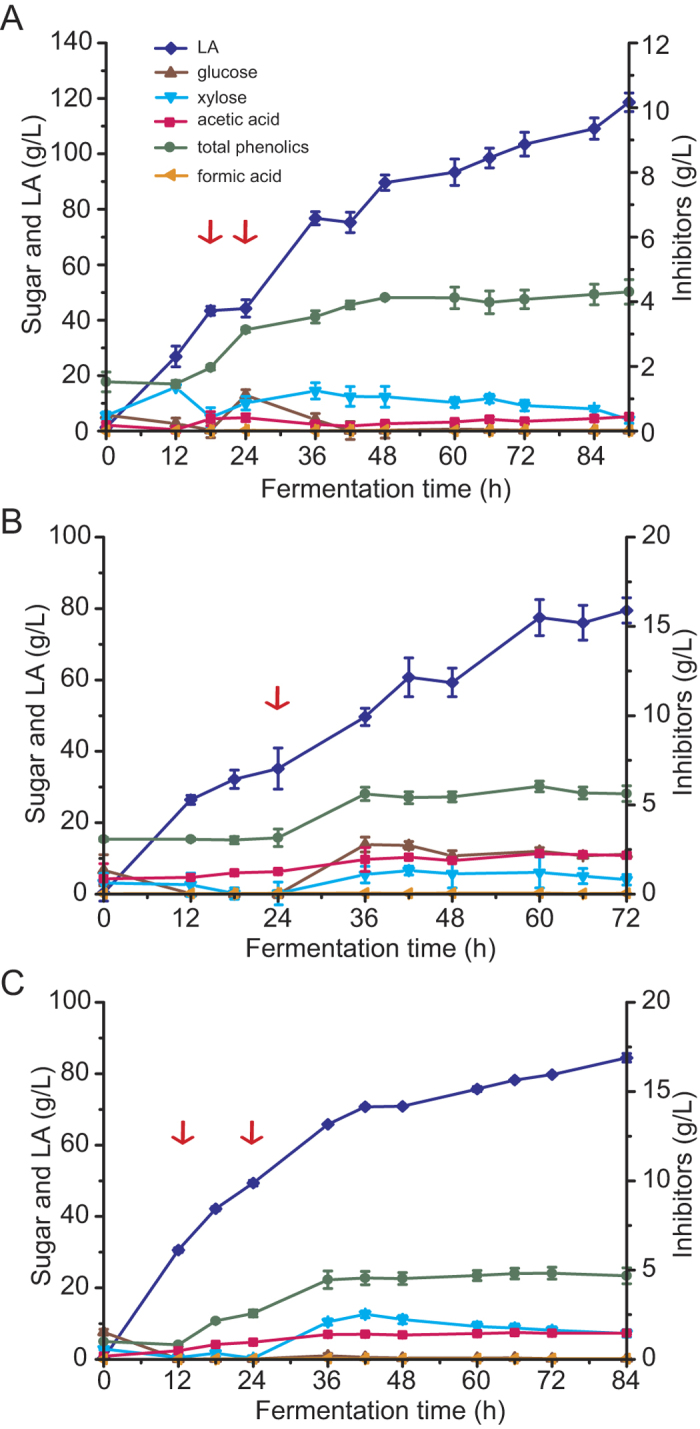
(A) Concentration of sugars (glucose and xylose), LA, and inhibitors (acetic acid, total phenolics, and formic acid) during fermentation of 8% pretreated and washed corncob substrate fed to 16% corncob from 18 to 24 h. (B) Fermentation of 8% pretreated and unwashed corncob substrate fed to 18.4% corncob at 24 h. (C) Fermentation of 4% pretreated and washed corncob substrate fed to 8% with pretreated and unwashed corncob at 12 h and fed to 16% with pretreated and unwashed corncob at 24 h. Substrate feeding is indicated by arrows. Blue diamond, LA; red square, acetic acid; green circle, total phenolics; brown triangle, glucose; cyan triangle, xylose; orange triangle, formic acid. Experiments were carried out in duplicate and error bars are shown. Where error bars are not visible, they are smaller than the size of the symbol used.
In order to fully eliminate the need for washing water, we performed fed-batch fermentation using NH3-H2O2-pretreated (1 day of NH3 treatment followed by 7 days of H2O2 treatment) and unwashed corncob as the substrate. However, in these experiments, we used a NaOH solution as the neutralizer, because the addition of CaCO3 to a bioreactor with high solid loading of NH3-H2O2-pretreated corncob led to a viscous fermentation culture, which was difficult to agitate. Fermentation was initiated with 8% unwashed corncob and the substrate was fed to 18.4% (w/w) at 24 h. The total phenolic concentration was 3.0 g/L during the initial stage (0 to 24 h) and increased following substrate feeding to 6.0 g/L. Liberated sugars were consumed rapidly during the initial stage; however, glucose and xylose accumulated in the culture post substrate feeding (Fig. 3B). These results indicate that inhibitors, such as phenolics, inhibited LA fermentation but did not inhibit sugar liberation from the corncob. Finally, the LA titer, yield, and productivity were 79.47 g/L, 0.43 g/g corncob, and 1.10 g/L/h, respectively (Fig. 3B). We hypothesize that initiating fermentation with a small amount of pretreated and washed corncob might increase cell activity and, therefore, enhance LA production efficiency. Thus, in the third experiment, fermentation was initiated with 4% (w/w) NH3-H2O2-pretreated and washed corncob. At this stage, sugars were consumed rapidly, LA was produced at a high rate (titer of 30.60 g/L and yield of 0.77 g/g corncob at 12 h, with productivity of 2.55 g/L/h during this period), and inhibitor concentrations were low (Fig. 3C). The first and second feedings with NH3-H2O2-pretreated and unwashed corncob were conducted at 12 h and 24 h; the substrate was fed to 8% and then to a final concentration of 16% (w/w). Following the feeding, LA was still produced rapidly (12 to 36 h) and the productivity was 1.47 g/L/h during this period. However, once the total phenolic concentration reached a maximum of 4.8 g/L at 36 h and was maintained at that level, LA productivity suddenly decreased to 0.39 g/L/h (36 to 84 h) and xylose accumulated in the culture to a concentration of 8 g/L. Fermentation was continued for 84 h with an LA titer, yield, and productivity of 84.46 g/L, 0.53 g/g corncob, and 1.01 g/L/h, respectively. LA titer and yield could be increased by extending fermentation time; however, this may result in lower overall productivity. In contrast, high-titer LA production was obtained from sulfuric acid-pretreated and biodetoxified corn stover by P. acidilactici DQ2. In their studies, acid-pretreated corn stover was subsequently detoxified by inoculation of Amorphotheca resinae ZN1 for 5 days in a separated fermentation process and then the biodetoxified corn stover was used for LA fermentation24,25. Thus, it should be considered to combine NH3-H2O2 pretreatment with biodetoxification to enhance LA fermentation by B. coagulans FL204 in our further study. It also should be noted that several additives can enhance fermentation performance. For example, 30 mM citrate buffer greatly influenced acetone-butanol-ethanol fermentation by Clostridium beijerinckii when corn stover hydrolysate was used as the substrate29. Therefore, useful LA fermentation additives that enhance the yield, titer, and productivity under our pretreatment and fermentation conditions should be identified. Table 3 compares and summarizes LA production from agro-biomass using different pretreatments (acid and alkaline) and fermentation methods (SSF and separate hydrolysis and fermentation [SHF]). This study reports one of the highest LA titers and yields produced from pretreated but unwashed or non-detoxified lignocellulosic materials. Therefore, NH3-H2O2 pretreatment might completely eliminate the need for washing water, at least when corncob is used as the substrate. However, it should also be noted that the LA titer and yield from NH3-H2O2-pretreated corncob are still very low compared with those from NH3-H2O2-pretreated and washed corncob. Moreover, NH3-H2O2 pretreatment is lengthier than the NaOH method, which will impact equipment size, throughput, and process economics.
Table 3. Summary of recent publications on lactic acid production from agro-biomass.
| Strain | Substrate | Pretreatment | Detoxification | Fermentation mode | Lactic acid |
Optical purity | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| titer (g/L) | yield (g/g) | productivity (g/L/h) | |||||||
| B. coagulans strain IPE22 | wheat straw (water insoluble solid after pretreatment) | sulfuric acid | — | SSF | 38.73 | 0.46a | 0.43 | N.D. | 23 |
| B. coagulans MXL-9 | corn fiber hydrolysate | sulfuric acid | — | SHF | 39 | 0.39a | 0.54 | 99% L-LA | 16 |
| B. coagulans DSM 2314 | wheat straw | lime | — | fed-batch SSF | 40.7 | 0.43a/0.81c | 0.68 | 97.2% L-LA | 21 |
| B. coagulans LA204 | corn stover | NaOH | water washing | fed-batch SSF | 97.59 | 0.68a | 1.63 | 97.9% L-LA | 12 |
| B. coagulans JI12 | hydrolysate of oil palm empty fruit bunch | sulfuric acid and phosphoric acid | — | fed-batch SHF | 137.5 | 0.98b | 4.4 | 99.5% L-LA | 22 |
| Bacillus sp. strain NL01 | corn stover hydrolysate | Steam explosion followed by enzyme saccharification | water washing | fed-batch SHF | 75 | 0.75b | 1.04 | N.D. | 32 |
| Lb. rhamnosus and Lb. brevis | corn stover | NaOH | water washing | fed-batch SSF | 60.3 | 0.7a | 0.58 | N.D. | 33 |
| Lb. plantarum NCIMB 8826 | corn stover | NaOH | — | fed-batch SSF | 61.4 | 0.77d | 0.32 | 99% D-LA | 34 |
| Lb. pentosus FL0421 | corn stover | NaOH | water washing | fed-batch SSF | 92.3 | 0.66a | 1.92 | 98.1% L-LA | 35 |
| P. acidilactici DQ2 | corn stover | dilute sulphuric acid | bio-detoxification | SSF | 101.9 | 0.77e | 1.06 | 63.4% L-LA | 25 |
| P. acidilactici TY112 | corn stover | dilute sulphuric acid | bio-detoxification | SSF | 104.4/77.76 | 0.72e/0.65e | 1.06 | 99.89% L-LA | 24,36 |
| P. acidilactici ZP26 | corn stover | dilute sulphuric acid | bio-detoxification | SSF | 76.76 | 0.58e | 1.02 | 99.32% D-LA | 36 |
| R. oryzae HZS6 | corncob hydrolysate | sulfuric acid | — | SHF | 77.2 | 0.80b | 0.99 | 100% L-LA | 37 |
| B. coagulans LA204 | corncob | NaOH/NH3-H2O2 | water washing | fed-batch SSF | 120.99/118.60 | 0.77a/0.74a | 1.37/1.32 | 98% L-LA | This study |
| NH3-H2O2 | — | 79.47 | 0.43a | 1.10 | |||||
ag/g total stover.
bg/g total sugar in the hydrolysate.
cg/g released total sugar.
dg/g used stover.
eg/g glucose from total cellulose. N.D.: not determined.
Methods
Raw material and substrate pretreatments
Corncob was harvested in 2014 in the Hubei province of China. After harvest, the corncob was cleaned, dried, and sieved using a 200-mesh. The raw corncob consisted of 37.26 ± 0.56% cellulose, 29.05 ± 0.04% hemicellulose, 19.60 ± 0.64% lignin, and 11.17 ± 0.01% ash. Two pretreatment methods were used in this study. The first method involved pretreating the corncob with 5% NaOH solution at 75 °C for 3 h using 20% (w/w) corncob loading. The resulting slurry was then washed with water until the pH decreased to 8.0 and then filtered to a moisture content of 25% (w/w). In the second method, the corncob was pretreated with 3% (w/w) ammonium hydroxide (NH3·H2O) for 1 day and then treated with 5% (w/w) H2O2 solution for 1 day or 7 days using 20% (w/w) corncob loading at room temperature. A portion of the NH3-H2O2-pretreated corncob was washed with water until the pH decreased to 8.0 and then filtered to a moisture content of 25% (w/w). The pH value of the remaining substrate was adjusted to 8.0 using a 50% (w/w) H2SO4 solution. The cellulase used in this study was Cellic CTec2 (Novozymes, Denmark), which contains cellulase, β-glucosidase, and xylanase activity. YEX medium (10 g/L xylose and 10 g/L yeast extract) was used for seed culturing. The analytical methods of the National Renewable Energy Laboratory (NREL) were used to determine the raw and pretreated material composition in terms of structural carbohydrates and lignin30.
LA fermentation from NaOH-pretreated corncob by SSF
The B. coagulans LA204 was inoculated into 200 mL YPX medium, pH6.0 at 50 °C and 100 rpm overnight. Under these conditions, the cells were in a logarithmic growth phase with a concentration of 1.6 × 107 colony forming units (CFU)/mL. The SSF process was established by inoculating 3 L of 8% (w/w) NaOH-pretreated and washed corncob, 10 g/L yeast extract, and 30 filter paper cellulase units (FPU)/g corncob with 300 mL seed culture in a 5-L bioreactor (BAOXING, Shanghai, China). LA fermentation by B. coagulans LA204 was performed at optimal conditions (50 °C and pH 6.0 [maintained by an automatic feed of 10 M NaOH solution or using excess CaCO3]), as previously reported12, for 48 h with agitation at 200 rpm. For the fed-batch experiments, 8% (w/w) NaOH-pretreated and washed corncob, 150 mL seed culture, and 30 FPU/g corncob of cellulase were used to establish fermentation at 50 °C for 18 h in a 1.5 L volume. Washed corncob was fed to 16% (w/w) and enzyme was fed from 18 h to 24 h to maintain a concentration of 30 FPU/g substrate; the total fermentation volume was 3 L. Samples were collected every 6 or 12 h during fermentation and the concentrations of LA, acetic acid, formic acid, glucose, xylose, and total phenolic compounds were determined and the yields and productivities were calculated. Fermentations were performed in duplicate under non-sterile conditions.
LA fermentation from NH3-H2O2-pretreated corncob by SSF
To establish the SSF process, a 3-L volume of 8% (w/w) NH3-H2O2-pretreated and unwashed corncob, 10 g/L yeast extract, and 30 FPU/g substrate of cellulase were inoculated with 300 mL seed culture in a 5-L bioreactor. Fermentations were carried out at 50 °C for 48 h with agitation at 200 rpm; the pH was maintained at 6.0 using an automatic feed of 10 M NaOH solution or using excess CaCO3. Two fed-batch fermentations were carried out to determine LA production ability. In the first fed-batch experiment, 8% NH3-H2O2-pretreated and unwashed corncob, 30 FPU/g substrate of cellulase, and 150 mL seed culture were used to initiate fermentation under the same fermentation conditions in a 1.5-L volume. The NH3-H2O2-pretreated and unwashed corncob was fed to 18.4% (w/w) and cellulase was fed to maintain 30 FPU/g substrate at 24 h; the final volume was 3 L. In the second fed-batch experiment, 4% (w/w) NH3-H2O2-pretreated and washed corncob, 30 FPU/g substrate of cellulase, and 150 mL seed culture were used to initiate fermentation under the same fermentation conditions in a 1.5-L volume. The NH3-H2O2-pretreated and unwashed corncob was fed to 8% (w/w) at 12 h and to 16% at 24 h and cellulase was fed to maintain 30 FPU/g substrate; the final volume was 3 L. In addition, fed-batch fermentations using NH3-H2O2-pretreated and washed corncob were also conducted using CaCO3 as the pH neutralizer; 8% NH3-H2O2-pretreated and washed corncob, 30 FPU/g substrate of cellulase, and 150 mL seed culture were used to initiate fermentation under the same fermentation conditions in a 1.5-L volume. The NH3-H2O2 pretreated and washed corncob was fed to 16% (w/w) and cellulase was fed to maintain 30 FPU/g substrate from 18 h to 24 h; the final volume was 3 L. Samples were collected every 6 or 12 h during fermentation and the concentrations of LA, acetic acid, formic acid, glucose, xylose, and total phenolic compounds were determined and the yields and productivities were calculated. These fermentations were conducted in duplicate under non-sterile condition. The crude HPLC data were included in Supplementary Figure 1.
Analysis of sugars, lactic acid, and inhibitors
The levels of glucose, xylose, LA, acetic acid, and formic acid in the samples were measured using high performance liquid chromatography (HPLC) with a Bio-Rad HPX-87H ion-exclusion column equipped with an Agilent 1200 and a RID-10A or SPD-20A detector. The mobile phase was 5 mM H2SO4 at a flow rate of 0.6 mL/min at 40 °C. All samples were centrifuged at 12,000 rpm for 2 min and filtered using nylon syringe filters (pore size 0.22 μm) prior to loading. All standards for HPLC analysis (glucose, xylose, lactic acid, acetic acid, and formic acid) were obtained from Sigma-Aldrich. The concentration of l-LA was determined using an SBA-40X biosensor (Shandong Biosensor Institute, China). The total content of phenolic compounds in the samples was determined using the Folin-Ciocalteu method31 with gallic acid as a calibration standard.
Additional Information
How to cite this article: Zhang, Z. et al. Comparison of high-titer lactic acid fermentation from NaOH- and NH3-H2O2-pretreated corncob by Bacillus coagulans using simultaneous saccharification and fermentation. Sci. Rep. 6, 37245; doi: 10.1038/srep37245 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors would like to thank the Special Fund for Agro-scientific Research in the Public Interest [No. 201503137] for the financial support.
Footnotes
Author Contributions Z.Z., Y.L. and N.P. conceived and designed the project; Z.Z. and Y.X. carried out the fermentations; X.H., X.L. and J.H. pretreated the materials; Z.Z., Z.R. and S.Z. analyzed the products and inhibitors; Z.Z., Y.L. and N.P. wrote the manuscript.
References
- Jiang D., Zhuang D., Fu J., Huang Y. & Wen K. Bioenergy potential from crop residues in China: Availability and distribution. Renew. Sust. Energ. Rev. 16, 1377–1382, doi: 10.1016/j.rser.2011.12.012 (2012). [DOI] [Google Scholar]
- Zhang C. et al. Microstructure regulation of super activated carbon from biomass source corncob with enhanced hydrogen uptake. Int. J. Hydrogen Energ. 38, 9243–9250 (2013). [Google Scholar]
- Oh S. J., Jung S. H. & Kim J. S. Co-production of furfural and acetic acid from corncob using ZnCl2 through fast pyrolysis in a fluidized bed reactor. Bioresour. Technol. 144, 172–178 (2013). [DOI] [PubMed] [Google Scholar]
- Li H. et al. A modified biphasic system for the dehydration of d-xylose into furfural using SO42−/TiO2−ZrO2/La3+ as a solid catalyst. Catal. Today 234, 251–256 (2014). [Google Scholar]
- Polman K. Review and analysis of renewable feedstocks for the production of commodity chemicals. Appl. Biochem. Biotechnol. 45, 709–722 (1994). [Google Scholar]
- Permadi D. A. & Kim Oanh N. T. Assessment of biomass open burning emissions in Indonesia and potential climate forcing impact. Atmos. Environ. 78, 250–258 (2013). [Google Scholar]
- Chaillou S., Bor Y. C., Batt C. A., Postma P. W. & Pouwels P. H. Molecular cloning and functional expression in Lactobacillus plantarum 80 of xylT, encoding the D-xylose-H+ symporter of Lactobacillus brevis. Appl. Environ. Microbiol. 64, 4720–4728 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J., Kreutzer R. & Smith D. Rice burning and asthma hospitalizations, Butte County, California, 1983–1992. Environ. Health Persp. 105, 980–985 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Rahman M. A., Tashiro Y. & Sonomoto K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J. Biotechnol. 156, 286–301 (2011). [DOI] [PubMed] [Google Scholar]
- Talebnia F., Karakashev D. & Angelidaki I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 101, 4744–4753 (2010). [DOI] [PubMed] [Google Scholar]
- Lloyd T. A. & Wyman C. E. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 96, 1967–1977 (2005). [DOI] [PubMed] [Google Scholar]
- Hu J. et al. High-titer lactic acid production from NaOH-pretreated corn stover by Bacillus coagulans LA204 using fed-batch simultaneous saccharification and fermentation under non-sterile condition. Bioresour. Technol. 182, 251–257 (2015). [DOI] [PubMed] [Google Scholar]
- Kim T. H. & Lee Y. Y. Pretreatment of corn stover by soaking in aqueous ammonia at moderate temperatures. Appl. Biochem. Biotechnol. 137–140 (2007). [DOI] [PubMed] [Google Scholar]
- Zhu Y., Lee Y. Y. & Elander R. T. Conversion of aqueous ammonia-treated corn stover to lactic acid by simultaneous saccharification and cofermentation. Appl. Biochem. Biotechnol. 137–140, 721–738 (2007). [DOI] [PubMed] [Google Scholar]
- Varga E., Schmidt A. S., Reczey K. & Thomsen A. B. Pretreatment of corn stover using wet oxidation to enhance enzymatic digestibility. Appl. Biochem. Biotechnol. 104, 37–50 (2003). [DOI] [PubMed] [Google Scholar]
- Bischoff K. M., Liu S., Hughes S. R. & Rich J. O. Fermentation of corn fiber hydrolysate to lactic acid by the moderate thermophile Bacillus coagulans. Biotechnol. Lett. 32, 823–828 (2010). [DOI] [PubMed] [Google Scholar]
- Liu T. et al. Coupling alkaline pre-extraction with alkaline-oxidative post-treatment of corn stover to enhance enzymatic hydrolysis and fermentability. Biotechnol. Biofuels 7, 48 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J. et al. Comparative transcriptome analysis reveals different molecular mechanisms of Bacillus coagulans 2-6 response to sodium lactate and calcium lactate during lactic acid production. PLoS One 10, e0124316 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C., Zhao X.-Q., Liu C.-G., Chen L.-J. & Bai F.-W. Prospective and development of butanol as an advanced biofuel. Biotechnol. Adv. 31, 1575–1584 (2013). [DOI] [PubMed] [Google Scholar]
- Su Y., Rhee M. S., Ingram L. O. & Shanmugam K. T. Physiological and fermentation properties of Bacillus coagulans and a mutant lacking fermentative lactate dehydrogenase activity. J. Ind. Microbiol. Biotechnol. 38, 441–450 (2011). [DOI] [PubMed] [Google Scholar]
- Maas R. H. et al. Lactic acid production from lime-treated wheat straw by Bacillus coagulans: neutralization of acid by fed-batch addition of alkaline substrate. Appl. Microbiol. Biotechnol. 78, 751–758 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L. et al. Conversion of acid hydrolysate of oil palm empty fruit bunch to L-lactic acid by newly isolated Bacillus coagulans JI12. Appl. Microbiol. Biotechnol. 97, 4831–4838 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen X., Luo J., Qi B. & Wan Y. An efficient process for lactic acid production from wheat straw by a newly isolated Bacillus coagulans strain IPE22. Bioresour. Technol. 158, 396–399 (2014). [DOI] [PubMed] [Google Scholar]
- Liu G., Sun J., Zhang J., Tu Y. & Bao J. High titer l-lactic acid production from corn stover with minimum wastewater generation and techno-economic evaluation based on Aspen plus modeling. Bioresour. Technol. 198, 803–810 (2015). [DOI] [PubMed] [Google Scholar]
- Zhao K. et al. Simultaneous saccharification and high titer lactic acid fermentation of corn stover using a newly isolated lactic acid bacterium Pediococcus acidilactici DQ2. Bioresour. Technol. 135, 481–489 (2013). [DOI] [PubMed] [Google Scholar]
- Toquero C. & Bolado S. Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour. Technol. 157, 68–76 (2014). [DOI] [PubMed] [Google Scholar]
- Kang K. E., Jeong G. T. & Park D. H. Pretreatment of rapeseed straw by sodium hydroxide. Bioproc. Biosyst. Eng. 35, 705–713 (2012). [DOI] [PubMed] [Google Scholar]
- Banerjee G., Car S., Scott-Craig J. S., Hodge D. B. & Walton J. D. Alkaline peroxide pretreatment of corn stover: effects of biomass, peroxide, and enzyme loading and composition on yields of glucose and xylose. Biotechnol. Biofuels 4, 16 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C. et al. The vital role of citrate buffer in acetone-butanol-ethanol (ABE) fermentation using corn stover and high-efficient product recovery by vapor stripping-vapor permeation (VSVP) process. Biotechnol. Biofuels 9, 146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluiter A. et al. Determination of structural carbohydrates and lignin in biomass. NREL Chemical Analysis and Testing Laboratory Analytical Procedures: LAP-002, NREL/TP- 510–42618. (2010). [Google Scholar]
- Singleton V. L., Orthofer R. & Lamuela-Raventós R. M. In Methods in Enzymology. 299 (ed Lester Packer) 152–178, Academic Press (1999). [Google Scholar]
- Ouyang J. et al. Open fermentative production of l-lactic acid by Bacillus sp. strain NL01 using lignocellulosic hydrolyzates as low-cost raw material. Bioresour. Technol. 135, 475–480 (2013). [DOI] [PubMed] [Google Scholar]
- Cui F., Li Y. & Wan C. Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour. Technol. 102, 1831–1836 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Enhanced D-lactic acid production from renewable resources using engineered Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 100, 279–288 (2015). [DOI] [PubMed] [Google Scholar]
- Hu J. et al. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 214, 74–80 (2016). [DOI] [PubMed] [Google Scholar]
- Yi X. et al. Engineering wild-type robust Pediococcus acidilactici strain for high titer L- and D-lactic acid production from corn stover feedstock. J. Biotechnol. 217, 112–121 (2016). [DOI] [PubMed] [Google Scholar]
- Bai D. M., Li S. Z., Liu Z. L. & Cui Z. F. Enhanced L-(+)-lactic acid production by an adapted strain of Rhizopus oryzae using corncob hydrolysate. Appl. Biochem. Biotechnol. 144, 79–85 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


