Abstract
The monotypic anuran family Ranixalidae is endemic to India, with a predominant distribution in the Western Ghats, a region that is home to several unique amphibian lineages. It is also one of the three ancient anuran families that diversified on the Indian landmass long before several larger radiations of extant frogs in this region. In recent years, ranixalids have been subjected to DNA barcoding and systematic studies. Nearly half of the presently recognized species in this family have been described over the last three years, along with recognition of a new genus to accommodate three previously known members. Our surveys in the Western Ghats further suggest the presence of undescribed diversity in this group, thereby increasing former diversity estimates. Based on rapid genetic identification using a mitochondrial gene, followed by phylogenetic analyses with an additional nuclear gene and detailed morphological studies including examination of museum specimens, new collections, and available literature, here we describe two new species belonging to the genus Indirana from the Western Ghats states of Karnataka and Kerala. We also provide new genetic and morphological data along with confirmed distribution records for all the species known prior to this study. This updated systematic revision of family Ranixalidae will facilitate future studies and provide vital information for conservation assessment of these relic frogs.
Introduction
Laurent [1] proposed the name ‘Indirana’ (presumably, Indi for Indian, and rana for frogs) for the Indian endemic members of subgenus Discodeles Boulenger 1920, and recognized it as a distinct genus. Subsequently, this radiation was recognized worthy of higher taxonomic considerations [2–4], formally being elevated to the rank of a distinct family Ranixalidae [5]. Until recently, Indirana Laurent 1986 was considered as the sole generic representative in this family. Three of the previously known members have been transferred to a new genus Sallywalkerana Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016 proposed within the same family. Together, the two genera presently include 14 recognized species, of which seven were described by European Zoologists [6–8] between 128–140 years ago, followed by one description each by Dubois [9], Padhye et al. [10] and Modak et al. [11], and four by Dahanukar et al. [12]. Two species described by Rao [13] are currently not recognized as valid [12]. Ever since their original descriptions, several members of this family have been assigned to various widespread genera such as Polypedates [6], Ixalus [6], Rana [7, 8], Rana (Discodeles) [13–15] and Philautus [13]. More than a century after the description of the oldest known species (Polypedates beddomii Günther 1876), taxonomic clarity initiated only with the proposal of a new genus Indirana (and Ranixalus Dubois 1986, later synonymized [16]). Several taxa were subsequently transferred to genus Indirana [17] resulting in stable generic placements. Commonly, ranixalids have been termed as Leaping frogs [18], a name which is appropriate for their behavior of leaping long distances after initiating salutatory locomotion, upon being approached.
Phylogenetic studies have provided molecular support for the distinct evolutionary position of Ranixalidae within the dominant Old World frog family Ranidae (sensu [19]) (e.g., [5, 19–27]). Roelants et al. [23] further highlighted members of this family as ancient frogs that originated prior to several recognized subfamilies in Ranidae (sensu [19]) (e.g., dicroglossids, ranids and rhacophorids), and showed its sister relationship with Micrixalidae. The first attempt to investigate the genetic diversity within this endemic family was made by Nair et al. [28–29] and subsequently by Modak et al. [30]. These studies suggested that many of the presently recognized species are likely to be complexes of several cryptic species, following which six new Indirana species have been described [10–12]. Even though a considerable amount of molecular data from populations across the Western Ghats became available in the recent years [10, 11, 28–30], species identity in most cases remained doubtful due to various persistent sources of confusion, including the status and unavailability of some type specimens [31]. A taxonomic review was provided by Nair et al. [32] followed by an integrated systematic revision by Dahanukar et al. [12]. However, none of these studies have been able to provide new collections for the poorly known Indirana longicrus and I. tenuilingua, for which the type material is considered long lost [33]. Both Indirana longicrus and I. tenuilingua were suggested to be considered as incertae sedis, the former under the order Anura and latter under the genus Indirana, until further information becomes available on these taxa [12].
The Western Ghats of India is a globally recognized biodiversity hotspot, which has been celebrated as being particularly rich in amphibian diversity and high family-level endemism [23, 31, 34, 35]. Two monotypic anuran families (Micrixalidae and Nasikabatrachidae) and twelve amphibian genera are exclusively found in the Western Ghats mountain ranges. Though Ranixalidae is largely regarded as a radiation restricted to the Western Ghats, the distribution of this endemic family is known to extend to the Eastern Ghats. The Western Ghats hotspot region has also witnessed a rapid increase in the number of new amphibian species over the past decade [36], with 223 species presently included in the amphibian inventory of this region [37, 38]. Further efforts to have a near-complete inventory of existing species diversity in various amphibian groups, especially ancient and endemic lineages such as ranixalids, is important for effective conservation of amphibians of the Western Ghats [36].
Our extensive fieldwork in the Western Ghats and particular sampling of ranixalid frogs from the entire region, prompted us to investigate the existing species diversity and genetic relationships within members of this family. Using samples from all the species described till date, we employed the DNA barcoding approach for rapid genetic identification, followed by phylogenetic analyses, to explore the possible existence of cryptic taxa. A combined understanding from the molecular and morphological data suggests that there are still several undescribed cryptic species in this morphologically conserved group, of which two are formally described here.
Materials and Methods
Ethics statement
This study was conducted with permissions and following guidelines from the responsible authorities in the State Forest Departments (Maharashtra, Karnataka, Kerala, Tamil Nadu), Ministry of Environment, Forest and Climate Change, Government of India. Our protocols of collection and research complied with the provisions of the Wildlife (Protection) Act 1972, Government of India. Specific methods of collection, euthanasia, tissue sampling and fixation used in our study followed the guidelines for use of live amphibians and reptiles in field research by the American Society of Ichthyologists and Herpetologists (ASIH), and were approved by the ethical committee of Department of Environmental Studies, University of Delhi.
Field surveys and specimen collection
Extensive field expeditions and sampling was carried out between the years 2000–2015 at 93 localities in the Western Ghats (S1 Table). Ranixalid frogs are typically forest dwelling and usually found in close association with streamside vegetation, damp leaf litter or rock cutting and crevices. Hence, our search efforts were focused on specific habitats inside primary and secondary forest areas. Several populations were collected from disturbed or fragmented habitats in secondary forests. Collections were primarily undertaken at night by locating calling males, or by opportunistic searches during both night and day. Sampled animals were euthanized in MS-222, fixed in 4% formalin and preserved in 70% ethanol. Prior to fixation, small portions of the thigh muscle or liver tissues were preserved in absolute ethanol for genetic studies. Tissue samples were stored at -20° C at Systematics Lab, University of Delhi (SDBDU). Type specimens are deposited at Zoological Survey of India–Western Ghats Field Research Centre (ZSI–WGRC) Kozhikode, and referred specimens are available at SDBDU. Geographical coordinates and elevation at each sampling locality were recorded using Garmin 76CSx. S1 Table provides the details of sampling localities along with the species recorded. Maps were prepared using QGIS (http://www.qgis.org).
Taxon sampling and DNA protocols
This study includes 269 ranixalid samples representing all known species in the family. One Nyctibatrachus sp. served as the outgroup taxon for phylogenetic analyses. For 134 ingroup individuals, sequence data was obtained from the GenBank (S2 Table). Genomic DNA was extracted from 135 new individuals, using Qiagen DNeasy tissue kit (Qiagen, Inc., Valencia, CA, USA) following manufacturer’s protocol. Fragments of the mitochondrial 16S rRNA (16S) gene (≈540 bp) from all samples and the nuclear Recombination activating gene 1 (Rag1) (≈555 bp) from 16 representative taxa were PCR-amplified using previously published primer sets 16Sar and 16Sbr [39], and Rag1-C and Rag1-E [35], respectively. Sequencing was performed on both strands using BigDye Terminator v3.1 Cycle Sequencing kit and ABI 3730 automated DNA sequencer (Applied Biosystems). Raw sequences were checked and assembled in ChromasPro v1.4 (Technelysium Pty Ltd.), and imported in MEGA 5.2 [40] for creating an alignment using the ClustalW option. Ambiguous sections in the non-coding mitochondrial DNA sequences were manually identified by eye and excluded from phylogenetic analyses. The coding nuclear gene sequences were optimized by comparison with amino acids. Sequences generated as part of this study are deposited in the GenBank under accession numbers KX966028–KX966179. Sequence details are provided in S2 Table.
Molecular analyses
Alignment of the 16S sequences contained 534 characters, of which 500 were used for constructing a Neighbour-Joining (NJ) tree using the Kimura 2-parameter (K2P) distance model in PAUP* 4.0b10 [41]. The NJ tree was only used as a tool to visualize genetic differences between species, as defined in this work, and to identify putative new species. Uncorrected pairwise genetic distances (p-distances) were computed in PAUP* 4.0b10 [41] using all characters. Based on the grouping of individuals in the NJ tree, intraspecific pairwise comparisons between individuals or populations of the same species, and interspecific pairwise comparisons between species, were calculated to assess levels of genetic divergence reliable for species delineation within the family [42]. Frequency of the genetic distances was plotted as a histogram to visualize the intraspecific and interspecific genetic variation.
In order to infer phylogenetic support for the known and candidate species, Maximum Likelihood (ML) tree was constructed using a combined mitochondrial and nuclear DNA dataset comprising of 1063 nucleotides for selected 16 ingroup taxa and the outgroup. The best-fit model of DNA evolution was determined by implementing the Akaike Information Criterion in ModelTest 3.4 [43]. Heuristic ML searches were performed in PAUP* 4.0b10 [41] using the GTR+G+I model with all parameters estimated. Clade support under the Maximum Likelihood criteria was evaluated with 1000 rapid bootstrap replicates, using RAxML 7.3.0 [44, 45] as implemented in raxmlGUI 1.1 [46]. Bayesian analyses were performed using the same model in MrBayes 3.1.2 [47]. Two parallel runs of four MCMC chains were executed for five million generations and the first 25% of trees sampled at a frequency of 100 generations were discarded as burn-in. Bayesian Posterior Probabilities (BPP) for the clades were estimated from a majority-rule consensus tree.
Adult morphology
Species identification was done using an integrative molecular and morphological approach. Molecular operational taxonomic units (MOTUs) were morphologically compared with all available types and new collections from the type localities. Sex and maturity were determined by examining the gonads through a small ventral incision. Only adult animals were used for morphometrics and morphological comparisons. For the convenience of discussion, ranixalid species were grouped as small (20–40 mm), medium (41–55 mm) and large (56–70 mm). Measurements and associated terminology follow Biju et al. [48, 49]. The measurements were taken to the nearest 0.1 mm, using a digital slide-caliper or a binocular microscope with a micrometer ocular. Digit number is represented by roman numerals I–V. The term shank refers to the lower part of the leg containing the tibia, and the term thigh for the upper part containing the femur. The webbing formulae follow Savage and Heyer [50] as modified by Myers and Duellman [51]. The extent of webbing relative to subarticular tubercles is described by numbering the tubercles 1–3, starting from the toe discs. All measurements provided in the taxonomy section are in millimeters. In the Material studied section, the following abbreviations after specimen numbers refer to: HT (holotype), PT (paratype), LT (lectotype) and RS (referred specimen). For museums and frequently used terms, abbreviations are as follows: BNHS (Bombay Natural History Society, Bombay, India), DU (University of Delhi, Delhi, India), MNHN (Muséum National d’Histoire Naturelle, Paris), NHM (Natural History Museum, formerly British Museum (Natural History)), BMNH, (British Museum (Natural History), London, United Kingdom), International Union for Conservation of Nature (IUCN), SDB (S D Biju), SG (Sonali Garg).
Nomenclatural acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature (ICZN), and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:C264EC2C-896A-40EB-AD23-1E0AC587BC1C. The electronic edition of this work was published in a journal with an ISSN, which has been archived and is available from the following digital repositories: PubMed Central, LOCKSS.
Results
DNA barcoding for rapid genetic identification
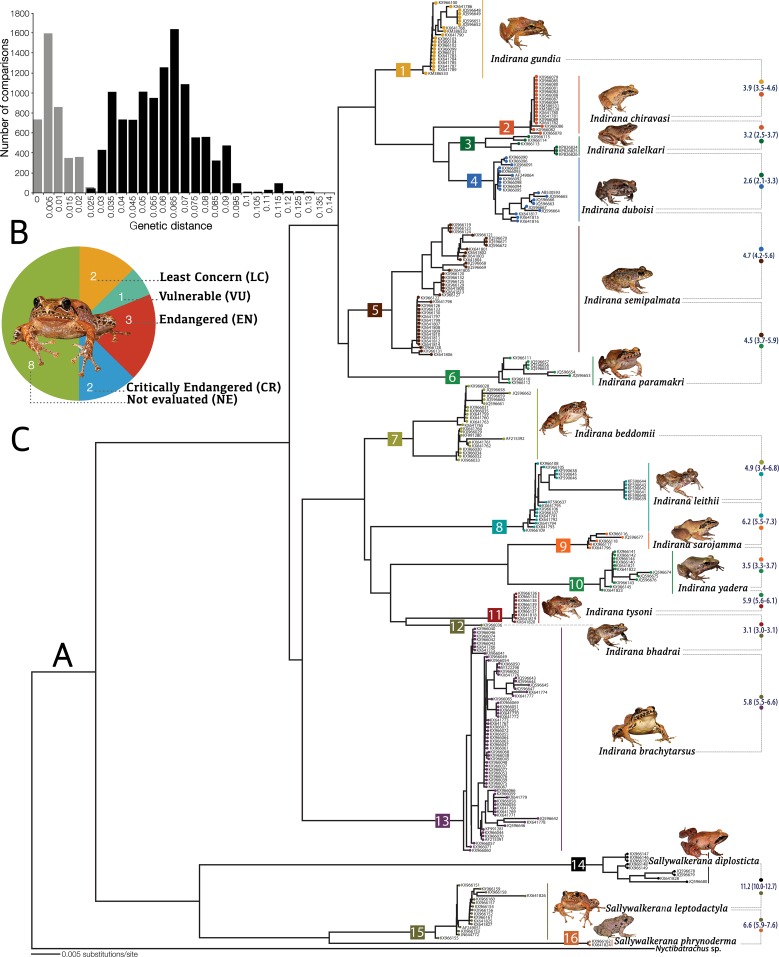
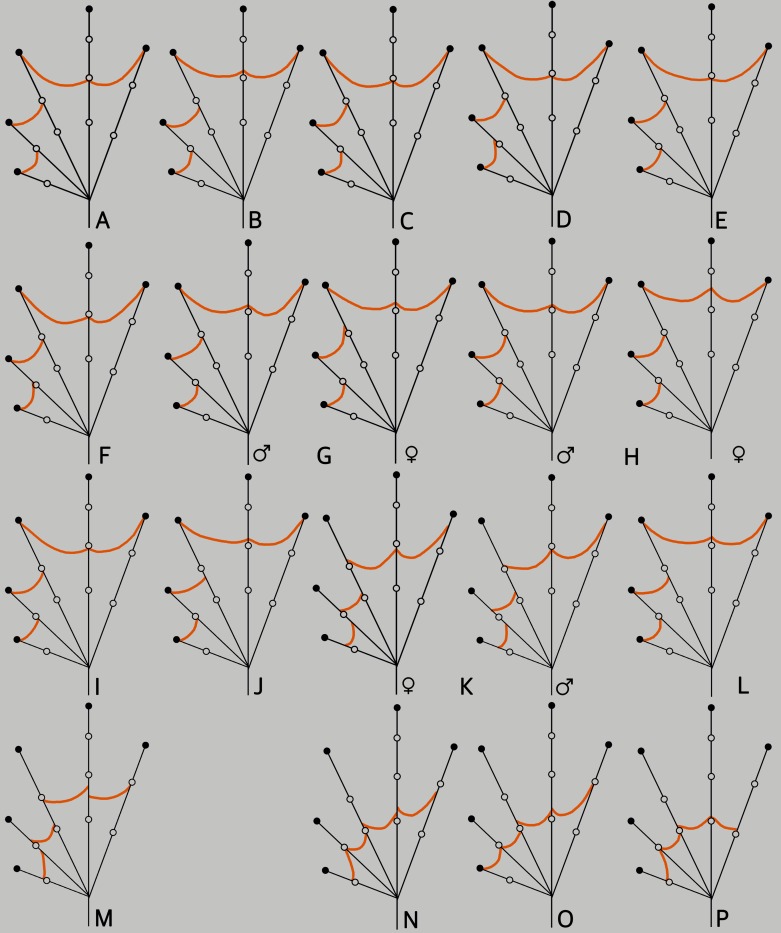
The Neighbour Joining (NJ) tree (Fig 1A) showed 16 major clusters corresponding to 11 known Indirana species: I. gundia (node 1), I. chiravasi (node 2), I. salelkari (node 3), I. duboisi (node 4), I. semipalmata (node 5), I. beddomii (node 7), I. leithii (node 8), I. sarojamma (node 9), I. yadera (node 10), I. tysoni (node 11), I. brachytarsus (node 13); two candidate species (nodes 6 and 12); and three known Sallywalkerana species: S. diplosticta (node 14), S. leptodactyla (node 15) and S. phrynoderma (node 16). Based on detailed morphological studies (see ‘Morphology and recognition of species‘), we formally describe two new Indirana species as–Indirana bhadrai sp. nov. and Indirana paramakri sp. nov.
Fig 1. DNA barcoding and status of IUCN assessment of ranixalid species of the Western Ghats.
(A) Neighbor-Joining (NJ) tree based on Kimura-2-parameter model for 16S mitochondrial gene sequences from 269 ranixalid samples, representing all previously known and newly recognized species from the Western Ghats. Terminal node labels indicate voucher numbers that are cross-referenced in S2 Table; clade numbers are indicated towards the internal nodes of the tree; mean and range of interspecific genetic distances (in percent) are shown between subsequent clades. (B) Frequency of intra (grey) and interspecific (black) uncorrected pairwise distances for 16S. (C) Number of ranixalid species in the different IUCN categories [66].
Uncorrected intraspecific genetic distance (S3 Table) was the highest at 3.5% for Indirana beddomii. Minimum interspecific distance was observed between two previously known species I. duboisi and I. salelkari, with the least divergence of 2.1% and mean divergence of 2.6% (S4 Table). In general, interspecific distances were low (minimum 2.1–2.7%) in the Indirana semipalmata group (see ‘Taxonomic grouping of species‘). Despite relatively lower genetic distances observed between some previously known species, we applied a conservative approach while delineating candidate species based on genetic distances. In the present study, we considered minimum 3.0% divergence value as reliable for delineating candidate species, since these values ensured no overlap between the maximum intraspecific pairwise distances within species and minimum interspecific distances between species (Fig 1B). Both the new species (nodes 6 and 12) we describe were delineated at a minimum and mean genetic distance of 3.0% from their closest relatives. These results were found to be largely consistent with genetic distances observed at species-level in other closely related groups in this region (e.g., [36, 49, 52, 53]). The maximum sequence divergence in our dataset was between Sallywalkerana diplosticta and S. phrynoderma (range 11.5–12.7%, mean 12.2%), and between S. diplosticta and S. leptodactyla (range 10.0–12.7%, mean 11.2%). For detailed genetic comparisons, see S3 and S4 Tables and the ‘Genetic divergence’ section of each species in the taxonomy section.
Phylogenetic analyses
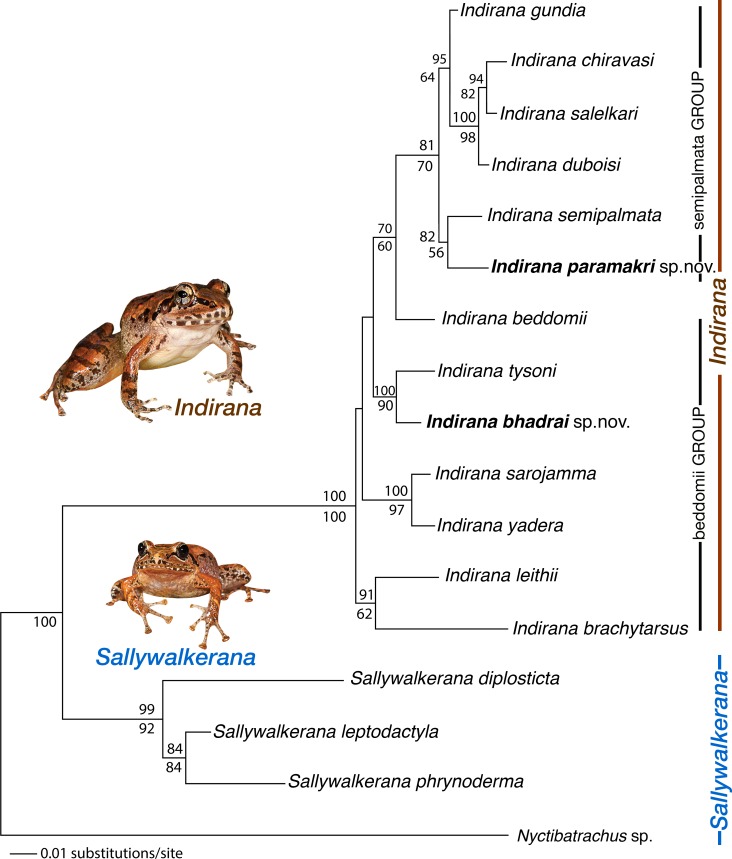
The Maximum Likelihood and Bayesian analyses recovered all the 16 recognized ranixalid species as distinct clades. Species-level relationships in the Maximum Likelihood and Bayesian frameworks were largely found to be in agreement (Fig 2). For the putative new species, sister relationship between Indirana bhadrai sp. nov. and I. tysoni (100 BPP, 90 BS) received high support, and the relationship of Indirana paramakri sp. nov. with I. semipalmata was recovered with moderate support (82 BPP, 56 BS). Members of the morphologically recognized Indirana beddomii group (I. beddomii, Indirana bhadrai sp. nov., I. brachytarsus, I. leithii, I. sarojamma, I. tysoni and I. yadera) appear to be paraphyletic but these relationships remained unresolved. On the hand, Indirana semipalmata group (including I. chiravasi, I. duboisi, I. gundia, Indirana paramakri sp. nov., I. salelkari and I. semipalmata) and genus Sallywalkerana (including S. diplosticta, S. leptodactyla and S. phrynoderma) formed monophyletic clades. However, the phylogenetic analyses performed in this study were only to infer molecular support for the new species and not for taxonomic grouping of species, which was primarily based on morphological characters (see ‘Taxonomic grouping of species’).
Fig 2. Maximum likelihood tree for a combined mitochondrial (16S rRNA) and nuclear (Rag1) DNA data set of 1063 bp showing phylogenetic relationships among 16 recognized ranixalid species and one outgroup species.
Bayesian Posterior Probabilities and RaxML Bootstrap values >50% are indicated above and below the branches, respectively. Morphological groups are referenced in the text.
Morphology and recognition of species
Detailed comparison of morphological data corroborated the molecular evidence in our study, thereby enabling proper identification of all sampled populations in the family Ranixalidae along with description of two new species in the genus Indirana.
The new species were compared with all available types, and with recently collected new specimens from the type localities. Since femoral gland is a secondary sexual character in males, which may be more prominent during the breeding season, its presence or absence is not considered as a diagnostic character in the present study. Among the specimens examined in our study, we observed femoral glands on the ventral surface of thighs in Indirana chiravasi, I. duboisi, Indirana paramakri sp. nov., I. salelkari, I. semipalmata, I. yadera and Sallywalkerana phrynoderma (S1 Fig), but they were absent in Indirana beddomii, I. brachytarsus, I. leithii, I. sarojamma, I. tysoni, Sallywalkerana diplosticta and S. leptodactyla. However, absence of femoral glands in all seasons could be confirmed only in Indirana brachytarsus, I. tysoni and Sallywalkerana diplosticta. Further studies will be required to confirm the presence or absence of femoral glands in ranixalid species, during the breeding as well as non-breeding season.
Taxonomic treatment
Family. Ranixalidae Dubois 1987
Type genus. Indirana Laurent 1986
Genus included. Indirana Laurent 1986, Sallywalkerana Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016
Common name. Leaping Frogs [18]
Etymology. The family name Ranixalidae is derived from two words, Rana meaning ‘frog’ in Latin, and the word ixalus, which is often used as a suffix in rhacophorid generic names.
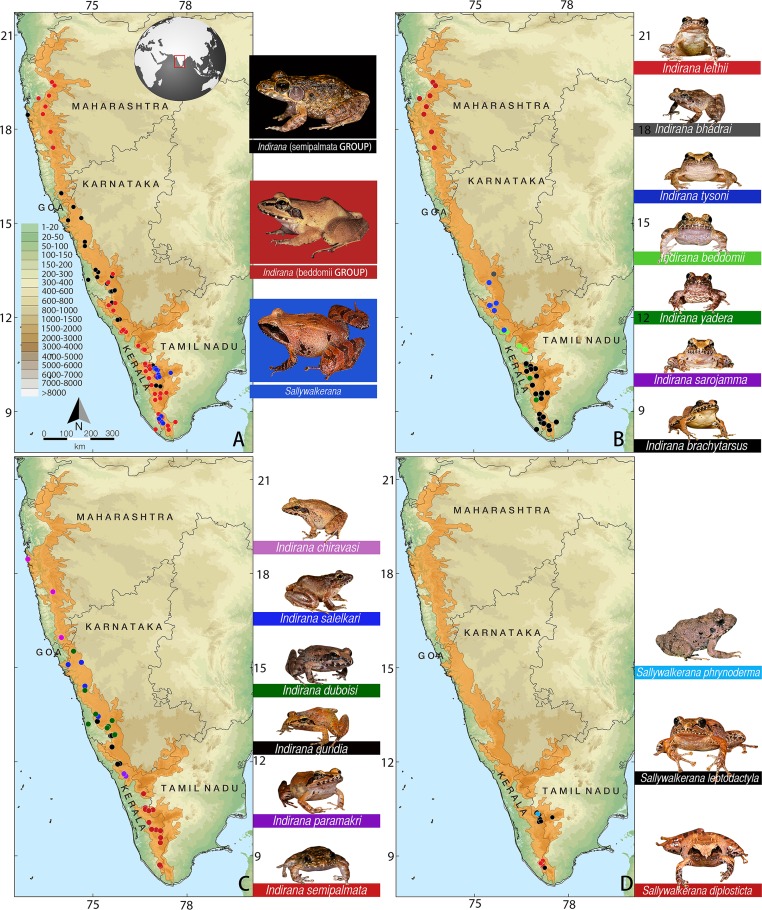
Distribution. Genus Indirana is endemic to Peninsular India, predominantly the Western Ghats states of Tamil Nadu, Kerala, Karnataka, Goa, Maharashtra, and southern Gujarat, and further extends up to Eastern Ghats in the state of Andhra Pradesh (Fig 3). Reports of Indirana frogs from Madhya Pradesh require verification. Genus Sallywalkerana is restricted to regions south of Palghat gap in the Western Ghats states of Kerala and Tamil Nadu (Fig 3).
Fig 3. Distribution localities of Leaping frog species reported from the Western Ghats of India in the present study.
(A) Sampling localities of all species. Species in genus Indirana are categorized into two morphological groups recognized in the study. (B) Sampling localities of seven species in the Indirana beddomii group. (C) Sampling localities of six species in the Indirana semipalmata group. (D) Sampling localities of three species in genus Sallywalkerana. Colors of sampling localities correspond with group/species labels on the right panel of each map. The Western Ghats biodiversity hotspot region is shaded orange.
Genus. Indirana Laurent 1986
Type species. Polypedates beddomii Günther 1876 [= Indirana beddomii (Günther 1876)]
Species included. Indirana beddomii (Günther 1876) Indirana bhadrai sp. nov., I. brachytarsus (Günther 1876), I. chiravasi Padhye Modak and Dahanukar 2014, I. duboisi Dahanukar, Modak, Krutha, Nameer, Padhye and Molur, 2016, I. gundia (Dubois 1986), I. leithii (Boulenger 1888), Indirana paramakri sp. nov., I. salelkari Modak, Dahanukar, Gosavi, and Padhye 2015, I. sarojamma Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016, I. semipalmata (Boulenger 1882), I. tysoni Dahanukar, Modak, Krutha, Nameer, Padhye and Molur, 2016, I. yadera Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016.
Note. Dahanukar et al. [12] have considered Rana (Discodeles) tenuilingua Rao 1937 [= Indirana tenuilingua (Rao 1937)] as incertae sedis under the genus Indirana, and Philautus longicrus Rao 1937 [= Indirana longicrus (Rao 1937)] as incertae sedis under the order Anura. Further studies will be required to confirm the taxonomic status of these two taxa (see ‘Discussion‘).
Common name. Indian Leaping Frogs
Etymology. The generic name Indirana is probably derived from two words, Indi for ‘Indian’, and Rana meaning ‘frog’ in Latin.
Distribution. The geographical range extends from Kiriparai (Kanyakumari district), Tamil Nadu state in the south, through the states of Kerala, Karnataka, Goa, Maharashtra, upto Surat Dangs in southern Gujarat [54], and further extends to Nallamala hills (Eastern Ghats) in Andhra Pradesh state [55]. Reports from Madhya Pradesh [56, 57] require further verification due to lack of voucher specimens. For distribution records of Indirana reported in the present study see Fig 3 and S1 Table.
Salient morphological characters. Male SVL 23.0–46.0 mm, female SVL 30.0–62.0 mm; pupil oval; presence of discontinuous dorsal skin folds or prominent skin warts; vomerine teeth present; tongue emarginated posteriorly with lingual papillae; tympanum distinct; supratympanic fold present; presence of a dark band that extends from the tip of snout or nostril, ending near the axilla on either sides of the head; tips of fingers and toes with discs having distinct dorsoterminal grooves; interdigital webbing absent between fingers; moderate to extensive webbing between toes.
Taxonomic grouping of species
Based on morphological similarities between species, we identify two major species groups in the genus Indirana–Indirana beddomii group and Indirana semipalmata group. For detailed morphological characters and species included, see the group-wise species accounts in the below section.
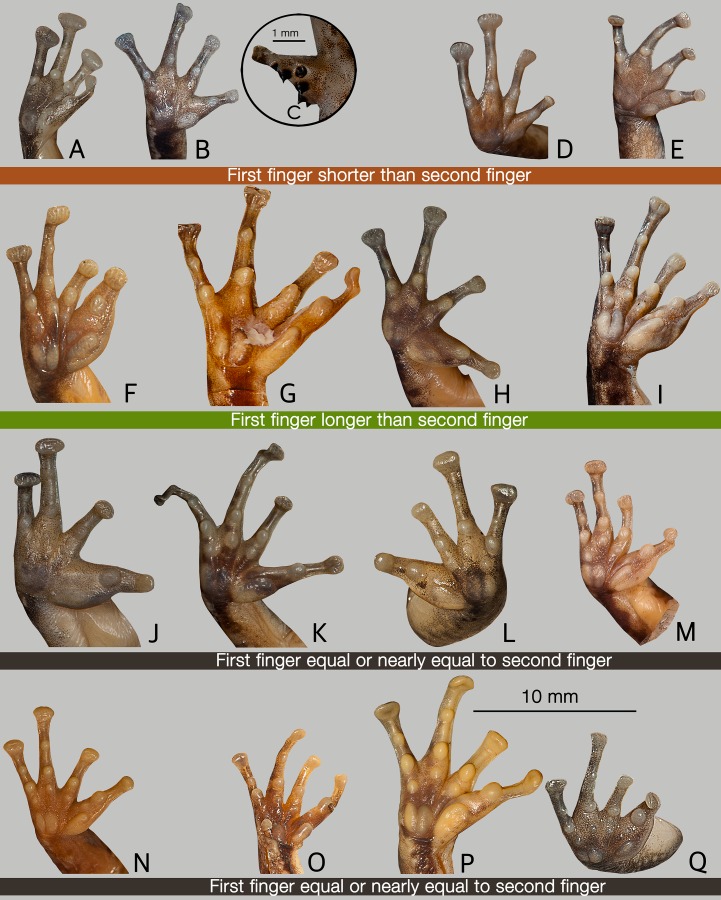
Indirana beddomii group. Morphologically this group can be distinguished from Indirana semipalmata group by the following suite of characters: small to large-sized adults (males: SVL 25.0–39.0 mm, females: SVL 31.0–63.0 mm), first finger longer than second finger, except in Indirana bhadrai sp. nov., I. brachytarsus and I. leithii (Fig 4), tympanum well-developed, smaller or nearly equal to horizontal diameter of eye (S2 Fig), and webbing on fourth toe extending up to the second subarticular tubercle on either side (Fig 5).
Fig 4. Ventral view of hands showing the relative length of first and second finger in ranixalid species.
(A–E) Species with first finger shorter than second finger. (A) Indirana leithii, SDBDU 2014.2515. (B, C) Sallywalkerana diplosticta, SDBDU 2015.2957. (B) Ventral surface. (C) Spinular projections on dorsal surface of first finger. (D) S. leptodactyla, SDBDU 2011.1058A. (E) S. phrynoderma, SDBDU 2002.1181. (F–I) Species with first finger longer than second finger. (F) Indirana beddomii, SDBDU 2010.225. (G) I. sarojamma, SDBDU 2002.334. (H) I. tysoni, SDBDU 2012.74. (I) I. yadera, SDBDU 2012.2744. (J–Q) Species with first finger equal or nearly to second finger. (J) I. brachytarsus, SDBDU 2015.2931. (K) Indirana bhadrai sp. nov., ZSI/WGRC/V/A887. (L) I. Chiravasi, SDBDU 2014.2483. (M) I. Duboisi, SDBDU 2003.1086. (N) I. Gundia, MNHN 1985.0633. (O) Indirana paramakri sp. nov., SDBDU 2005.3741. (P) I. Salelkari, SDBDU 2011.1330. (Q) I. Semipalmata, SDBDU 2015.3035.
Fig 5. Schematic illustration of foot webbing in ranixalid species.
(A–G) Indirana beddomii group. (A) I. beddomii, lectotype of Polypedates beddomii, NHM 74.4.29.208 (ex BMNH 1947.2.27.72), female. (B) Indirana bhadrai sp. nov., holotype, ZSI/WGRC/V/A887, female. (C) I. brachytarsus, lectotype of Polypedates brachytarsus, NHM 74.4.29.1307 (ex BMNH 1947.2.27.92), female. (D) Indirana leithii, holotype of Rana leithii, NHM 69.8.28.50 (ex BMNH 1947.2.28.17), female. (E) Indirana sarojamma, SDBDU 2002.516, female. (F) I. tysoni, SDBDU 2012.73, female. (G) I. yadera, SDBDU 2012.2744, male (left), SDBDU 2015.2984, female (right). (H–M) Indirana semipalmata group. (H) I. chiravasi, SDBDU 2014.2423, male (left), SDBDU 2015.3087, female (right). (I) I. duboisi, SDBDU 2003.1086, male. (J) I. gundia, holotype of Ranixalus gundia, MNHN 1985.0633, male. (K) Indirana paramakri sp. nov., SDBDU 2015.3741, male (left), ZSI/WGRC/V/A888, female (right). (L) I. salelkari, SDBDU 2011.1330, female. (M) I. semipalmata, lectotype of Rana semipalmata, NHM 74.4.29.605 (ex BMNH 1947.2.29.50), female. (N–P) Genus Sallywalkerana. (N) S. diplosticta, lectotype of Ixalus diplostictus, NHM 74.4.29.1412 (ex BMNH 1947.2.2.21), female. (O) Sallywalkerana leptodactyla, lectotype of Rana leptodactyla, NHM 74.4.29.593 (ex BMNH 1947.2.29.39), female. (P) Sallywalkerana phrynoderma, lectotype of Rana phrynoderma, NHM 82.2.10.21 (ex BMNH 1947.2.3.8), female.
Members included. Indirana beddomii, Indirana bhadrai sp. nov., I. brachytarsus, I. leithii, I. sarojamma, I. tysoni and I. yadera.
Indirana beddomii (Günther 1876)
Beddome's Leaping Frog [58]
(Figs 1–6; S2–S4 Figs; S1–S4 Tables; S1 File)
Fig 6. Indirana beddomii group in life.
(A–B) I. beddomii. (A) An adult male (SDBDU 2010.225), from Kakkayam. (B) An adult female (SDBDU 2011.960), from Silent Valley. (C) Indirana bhadrai, holotype, an adult female (ZSI/WGRC/V/A887), from Muthodi. (D–H) I. brachytarsus. (D) An adult female (not preserved), from Ponmudi. (E) An adult male (SDBDU 2011.541), from Parambikulam. (F) An adult male (SDBDU 2006.4800), from Anchuruli. (G) An adult female (SDBDU 2011.280), from Pandimotta. (H) An adult male (SDBDU 2015.2995), from Upper Manalar. (I, J) I. leithii. (I) An adult female (SDBDU 2014.2514), from Matheran. (J) An adult male, (SDBDU 2011.1095), from Bhimashankar. (K) I. sarojamma, an adult female (SDBDU 2002.516) from Chathankod–Bonnacaud. (L–N) I. tysoni. (L, M) An adult female (SDBDU 2012.73), from Coorg. (L) Dorsolateral view. (M) Frontolateral view. (N) An adult male (SDBDU 2012.2228), from Coorg. (O–R) I. yadera. (O) An adult male (SDBDU 2012.2744), from Methooty. (P, Q) An adult male (SDBDU 2015.2984), from Kozhikana. (P) Frontal view. (Q) Dorsolateral view. (R) An adult female (SDBDU 2015.3155), from Vazhachal.
Original name and description. Polypedates beddomii Günther 1876. Third report on collections of Indian reptiles obtained by the British Museum, Proceedings of the Zoological Society of London, 1876 “1875”: 571–572. Lectotype. NHM 74.4.29.208 (ex BMNH 1947.2.27.72), designated by Dahanukar et al. [12], adult female, from “Malabar”, figured in Günther 1876: LXIII. Fig B. Type locality. “Malabar”, India, according to original description. Current status of specific name. Valid name, as Indirana beddomii (Günther 1876).
Comments. The original description [6] mentions several specimens from four different localities—Malabar, Travancore, Anamallays and Sevagherry, all from Col. Beddome’s collection. We examined all the specimens (five males, six females and nine sub-adults) available at NHM, and found NHM 74.4.29.208 (ex BMNH 1947.2.27.72) from “Malabar” to be in agreement with the original description, with respect to the snout-vent size (“Length of body 55 mm”), snout shape (“rather obtuse”) and webbing on foot (“toes two thirds webbed”). It was also found to be in well-preserved condition (S4 Fig). Dahanukar et al. [12] designated this specimen as the lectotype.
Genetic divergence. Intraspecific genetic variation within populations of I. beddomii is 1.2 ± 0.9% (range 0–3.5%, N = 22) for 16S mitochondrial gene sequences. Indirana beddomii is closely related to members of the Indirana beddomii group (Figs 1 and 2); differs from I. bhadrai by mean genetic divergence of 4.2 ± 0.3% (range 3.7–5.0%, N = 22); from I. brachytarsus by mean genetic divergence of 6.3 ± 0.4% (range 5.5–7.7%, N = 1408); from I. leithii by mean genetic divergence of 4.9 ± 0.7% (range 3.4–6.8%, N = 440); from I. sarojamma by mean genetic divergence of 5.5 ± 0.4% (range 5.0–7.0%, N = 110); from I. tysoni by mean genetic divergence of 4.7 ± 0.5% (range 4.0–5.9%, N = 198); and from I. yadera by mean genetic divergence of 6.2 ± 0.5% (range 5.5–8.5%, N = 264) (S3 and S4 Tables).
Distribution and natural history. Indirana beddomii is currently known only from the state of Kerala in the southern Western Ghats. In the present study, we found this species north of Palghat gap at Kakkayam, Settukunnu and Suganthagiri; and south of Palghat gap at Sairandhri (Silent Valley), Kuddam (Siruvani) and Pattiar (Siruvani) (Fig 3 and S1 Table). Specimens were located during night searches (between 17:00–19:00 hours) and were found either on earthen cutting inside evergreen forest or on forest floor in secondary forests. For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
For morphological comparison, description of lectotype, secondary sexual characters, variations, and list of specimens examined, see S1 File.
Indirana bhadrai sp. nov.
urn:lsid:zoobank.org:act:9E20EF06-F9A4-4528-84C0-69C9CB4E0D91
Bhadra Leaping Frog
(Figs 1–7; Table 1; S2 and S3 Figs; S1–S4 Tables)
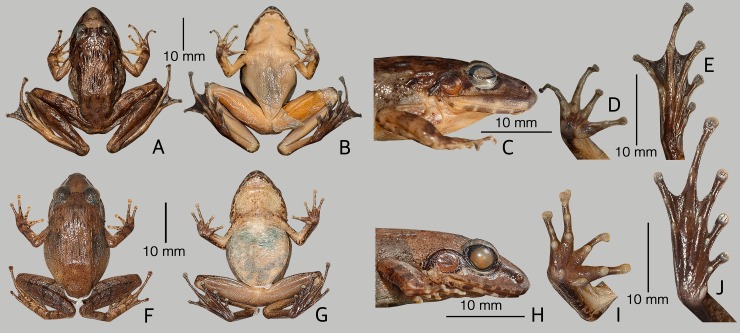
Fig 7. Two new Indirana species from the Western Ghats.
(A–E) Holotype of Indirana bhadrai sp. nov., ZSI/WGRC/V/A887, female, in preservation. (F–J) Holotype of Indirana paramakri sp. nov., ZSI/WGRC/V/A888, female, in preservation. From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot.
Table 1. Measurements (in mm) of the type and referred specimens of two new species of Indirana.
The range, mean value, and standard deviation are given for each parameter.
| Indirana bhadrai sp. nov. | Indirana paramakri sp. nov. | |||
|---|---|---|---|---|
| Male (N = 1) | Female (N = 1) | Male (N = 2) | Female (N = 2) | |
| SVL | 30.2 | 38.7 | 27.4–30.1 (28.8 ± 1.9) | 32.9–34.1 (33.5 ± 0.8) |
| HW | 11.8 | 15.0 | 11.3–11.7 (11.5 ± 0.3) | 13.0–13.1 (13.1 ± 0.1) |
| HL | 11.5 | 14.9 | 12.0–12.3 (12.2 ± 0.2) | 13.6–13.8 (13.7 ± 0.1) |
| TYD | 2.4 | 2.8 | 2.8–3.5 (3.2 ± 0.5) | 3.1–3.2 (3.2 ± 0.1) |
| SL | 5.4 | 6.4 | 4.5–5.2 (4.9 ± 0.5) | 5.6–5.8 (5.7 ± 0.1) |
| EL | 3.9 | 4.5 | 3.4–3.8 (3.6 ± 0.3) | 3.9–4.1 (4.0 ± 0.1) |
| MN | 10.2 | 11.6 | 9.1–9.6 (9.4 ± 0.4) | 11.4–11.7 (11.6 ± 0.2) |
| EN | 2.3 | 3.7 | 2.3–2.6 (2.5 ± 0.2) | 2.4–3.1 (2.8 ± 0.5) |
| NS | 1.9 | 2.7 | 1.5–1.6 (1.6 ± 0.1) | 1.8–2.2 (2.0 ± 0.3) |
| IUE | 3.1 | 3.4 | 2.8–3.2 (3.0 ± 0.3) | 3.2–3.4 (3.3 ± 0.1) |
| UEW | 2.1 | 3.0 | 1.9–2.1 (2.0 ± 0.1) | 2.3–2.7 (2.5 ± 0.3) |
| FAL | 6.7 | 7.9 | 5.4–6.6 (6.0 ± 0.8) | 6.6–6.8 (6.7 ± 0.1) |
| HAL | 8.1 | 11.2 | 6.6–7.9 (7.3 ± 0.9) | 7.4–7.8 (7.6 ± 0.3) |
| FIL | 5.1 | 6.1 | 3.7–3.8 (3.8 ± 0.1) | 3.8–4.1 (4.0 ± 0.2) |
| FIIL | 5.2 | 6.2 | 3.7–3.7 (3.7 ± 0.0) | 3.7–4.0 (3.9 ± 0.2) |
| FIIIL | 6.2 | 7.3 | 3.5–4.3 (3.9 ± 0.6) | 4.3–5.2 (4.8 ± 0.6) |
| FIVL | 4.1 | 5.3 | 2.9–3.0 (3.0 ± 0.1) | 3.3–3.4 (3.4 ± 0.1) |
| TL | 18.3 | 22.5 | 14.9–16.8 (15.9 ± 1.3) | 16.4–16.7 (16.6 ± 0.2) |
| ShL | 20.1 | 25.0 | 15.9–17.3 (16.6 ± 1.0) | 17.9–18.6 (18.3 ± 0.5) |
| FOL | 18.9 | 21.9 | 14.2–15.6 (14.9±1.0) | 16.2–16.8 (16.5 ± 0.4) |
Etymology. The species name bhadrai is a noun in the genitive case, derived from Bhadra Wildlife Sanctuary, the region in which the type locality Muthodi forest is situated.
Holotype. ZSI/WGRC/V/A887, an adult female, from Muthodi forest, Bhadra Wildlife Sanctuary (12.2201°N 75.6557°E, 1176 m asl), Chikmagalur district, Karnataka state, collected by SDB and SG on 30 July 2012.
Referred specimen. SDBDU 2012.1471, an adult male, collected along with the holotype.
Comparison. Based on the overall morphology, Indirana bhadrai sp. nov. could be confused with I. beddomii, I. brachytarsus, I. leithii, I. sarojamma, I. tysoni and I. yadera within the I. beddomii group. However, I. bhadrai differs from all these species by its snout nearly pointed in dorsal view (vs. rounded in I. beddomii, I. brachytarsus and I. leithii; nearly truncate in I. sarojamma; sub-ovoid in I. tysoni and I. yadera), snout nearly acute in lateral view (vs. rounded in I. beddomii, I. brachytarsus, I. leithii and I. tysoni; obtuse in I. sarojamma), third toe webbing not extending up to the second subarticular tubercle on the inside, I1–2II1–21/4III1–3–IV3––1V (vs. up to the second subarticular tubercle in I. beddomii, I. brachytarsus, I. leithii and I. tysoni; and beyond the second subarticular tubercle in I. yadera, I1–2II1–2−III1–3−IV3−–1V) (Fig 5), first finger nearly equal to second finger, male FIL 5.1 mm, N = 1, female FIL 6.1 mm, N = 1 vs. male FIIL 5.2 mm, N = 1, female FIIL 6.2 mm, N = 1 (vs. longer in I. beddomii, I. sarojamma, I. tysoni and I. yadera: male FIL 4.1 ± 1.3 mm, N = 2, female FIL 6.9 ± 0.6 mm, N = 5 vs. male FIIL 3.1 ± 1.0 mm, N = 2, female FIIL 5.5 ± 0.3 mm, N = 5 in I. beddomii, male FIL 4.9 ± 0.1 mm, N = 2, female FIL 8.1 mm, N = 1 vs. male FIIL 3.8 ± 0.4 mm, N = 2, female FIIL 6.9 mm, N = 1 in I. sarojamma, male FIL 4.6 ± 0.4 mm, N = 4, female FIL 7.1 ± 0.3 mm, N = 2 vs. male FIIL 3.4 ± 0.1 mm, N = 4, female FIIL 5.9 ± 0.4 mm, N = 2 in I. tysoni, and male FIL 5.4 ± 0.5 mm, N = 3, female FIL 7.5 mm, N = 1 vs. male FIIL 3.9 ± 0.4 mm, N = 3, female FIIL 6.2 mm, N = 1 in I. yadera; and shorter in I. leithii: male FIL 2.6 ± 0.2 mm, N = 3, female FIL 3.4 ± 0.1 mm, N = 5 vs. male FIIL 3.8 ± 0.2 mm, N = 3, female FIIL 4.5 ± 0.3 mm, N = 5) (Fig 4), loreal region acute (vs. obtuse in I. beddomii and indistinct in I. leithii), and smaller adult size, male: SVL 30.2 mm, N = 1, female: SVL 38.7 mm, N = 1 (vs. larger, male: SVL 36.4 ± 2.6 mm, N = 2, female: SVL 61.2 mm, N = 1 in I. sarojamma). For more differences with I. beddomii see ‘Comparison’ of that species (S1 File).
For better clarity, we compare this new species with all other currently known species in this genus. Indirana bhadrai differs from the following members of Indirana semipalmata group: I. gundia, I. paramakri and I. semipalmata by its snout nearly pointed in dorsal view (vs. rounded in I. gundia and I. semipalmata, and sub-ovoid in I. paramakri), snout nearly acute in lateral view (vs. rounded in I. gundia and I. semipalmata), loreal region acute (vs. obtuse in I. gundia and I. semipalmata), first toe webbing extending up to the disc, I1–2II1–21/4III1–3−IV3−–1V (vs. below in I. gundia, I1–2II1–21/4III1–3−IV3−–1V; and just above the first subarticular tubercle in I. semipalmata, I2−–2+II2−–3−III2–31/4IV31/2–2V, and I. paramakri, I12/3–2+II13/4–23/4III2−–3IV3–1+V); differs from I. chiravasi and I. salelkari, by its snout nearly pointed in dorsal view (vs. sub-ovoid in I. chiravasi, and rounded in I. salelkari), snout nearly acute in lateral view (vs. obtuse in I. chiravasi, and rounded in I. salelkari), and loreal region acute (vs. obtuse in I. chiravasi and I. salelkari); specifically differs from I. chiravasi by its third toe webbing below the first subarticular tubercle on the inside, I1–2II1–21/4III1–3−IV3−–1V (vs. extending up to first subarticular tubercle, I1–2II1–2III1–3−IV3−–1V); differs from I. duboisi by its snout sub-ovoid in dorsal view (vs. nearly pointed), snout nearly acute in lateral view (vs. rounded), loreal region acute (vs. obtuse), and webbing between first, second and third toes not extending up to the disc on the outside, I12/3–2+II13/4–23/4III2−–3IV3–1+V (vs. upto the disc, I1–2II1–2III1–3−IV3−–1V) (Fig 5).
Furthermore, Indirana bhadrai differs from members of the genus Sallywalkerana by its larger adult snout-vent size, male: SVL 30.2 mm, N = 1, female: SVL 38.7 mm, N = 1 (vs. smaller), first finger equal or nearly equal to second finger (vs. first finger shorter than second), and third toe webbing extending up to second subarticular tubercle on either side, I1–2II1–21/4III1–3−IV3−–1V (vs. well below) (Fig 5).
Genetic divergence. Intraspecific genetic variation within populations of Indirana bhadrai could not be calculated since the present study included a single sample of this species. Based on 16S mitochondrial gene sequences and phylogenetic analyses, I. bhadrai is closely related to members of the I. beddomii group (Figs 1 and 2); differs from I. brachytarsus by mean genetic divergence of 5.8 ± 0.2% (range 5.5–6.6%, N = 64); from I. leithii by mean genetic divergence of 4.7 ± 0.7% (range 3.9–5.8%, N = 20); from I. sarojamma by mean genetic divergence of 5.1 ± 0.4% (range 4.9–5.7%, N = 5); from I. tysoni by mean genetic divergence of 3.1 ± 0.1% (range 3.0–3.1%, N = 9); and from I. yadera by mean genetic divergence of 5.6 ± 0.2% (range 5.3–6.1%, N = 12) (S3 and S4 Tables). See I. beddomii for comparison with that species.
Description of holotype (measurements in mm). Adult female (SVL 38.7); head small, its length (HL 14.9) subequal to the width (HW 15.0), flat above; snout nearly pointed in dorsal view, nearly acute in lateral view, its length (SL 6.4) longer than horizontal diameter of eye (EL 4.5); loreal region acute and concave with rounded canthus rostralis; interorbital space flat, wider (IUE 3.4) than upper eyelid (UEW 3.0), and narrower than internarial distance (IN 4.1); nostrils closer to tip of snout (NS 2.7) than eye (EN 3.7); tympanum (TYD 2.8) 62% of eye diameter (EL 4.5). Forelimbs (FAL 7.9) shorter than hand length (HAL 11.2), finger length formula: IV<I<II<III, finger discs moderately wide compared to finger width (FDI 1.1, FWI 0.6; FDII 0.9, FWII 0.5; FDIII 1.4, FWIII 0.6; FDIV 1.4, FWIV 0.5). Thigh length (TL 22.5) shorter than shank (SHL 25.0), and longer than foot (FOL 21.9), toe discs wide compared to toe width (TDI 1.4, TWI 0.6; TDII 1.7, TWII 0.5; TDIII 1.7, TWIII 0.6; TDIV 1.7, TWIV 0.6; TDV 1.2, TWV 0.5), foot webbing: I1–2II1–21/4III1–3−IV3−–1V (Fig 5).
Skin of snout and between eyes shagreened, upper eyelids shagreened to sparsely granular; posterior part of dorsum sparsely granular; dorsum with a few longitudinal discontinuous folds, lateral surface granular; anterior and posterior parts of flanks shagreened to sparsely granular; dorsal surface of forelimbs shagreened to finely granular. Ventral surfaces of throat and chest smooth, abdomen and posterior parts of thigh granular.
Colour of holotype. In life. Dorsum light brown with irregular dark brown blotches especially along the dorsal skin folds; a dark greyish-brown band between the eyes which continues over the upper eyelid; snout lighter in colour than dorsum; margins of upper and lower jaw with alternate dark brown and cream coloured cross-bars (Fig 6); a dark blackish-brown band extending from the from tip of snout through the lower margin of eye, widening behind the eye and over the tympanum, and ending near the armpit on either sides of the head; tympanum dark greyish-brown; forelimbs (including fingers) and hindlimbs (including toes) light brown with dark brown transverse bands; anterior and posterior parts of flank light yellowish-brown. Ventral surface light grey with dark grey spots. In preservation. Dorsum brown with irregular dark-brown blotches, especially along with dorsal skin folds; snout light brown; margins of upper and lower jaw with alternate dark brown and cream coloured cross-bars (Fig 7); a dark brown band extending from the snout through the lower margin of eye, widening behind the eye and over the tympanum, and ending near the armpit on either sides of the head; tympanum reddish-brown; forelimbs and hindlimbs brown with dark brown cross-bands. Ventral surfaces light creamish-brown (Fig 7).
Variation. See Table 1 for morphometric data from an adult male and female.
Distribution and natural history. Indirana bhadrai is currently known only from its type locality Muthodi forest, Bhadra Wildlife Sanctuary, located north of Palghat Gap in the Western Ghats state of Karnataka (Fig 3). Animals were found on leaf litter in a secondary forest, between 18:00–19:00 hours.
Indirana brachytarsus (Günther 1876)
Short-legged Leaping Frog [58]
(Figs 1–6; S1–S4 Figs; S1–S4 Tables; S1 File)
Original name and description. Polypedates brachytarsus Günther 1876. Third report on collections of Indian reptiles obtained by the British Museum, Proceedings of the Zoological Society of London, 1876 “1875”: 572. Lectotype. NHM 74.4.29.1307 (ex BMNH 1947.2.27.92), designated by Inger et al. [59], adult female, from “Anamallays”. Type locality. “Anamallays”, India, according to original description. Current status of specific name. Valid name, as Indirana brachytarsus (Günther 1876).
Comments. Originally described by Günther [6] as Polypedates brachytarsus from Anamallays and Sevagherry, this species was placed under the synonymy of Rana beddomii [7], and later resurrected as a distinct species [59]. Inger et al. [59] also designated NHM 74.4.29.1307 (ex BMNH 1947.2.27.92) (from Anamallays) as the lectotype of Polypedates brachytarsus (S4 Fig), but cited the voucher number as “BMNH 1947.2.27.1307”. In order to avoid confusion, we confirm the voucher number of the lectotype to be NHM 74.4.29.1307 (ex BMNH 1947.2.27.92) as per the bottle label and the NHM catalogue.
Genetic divergence. Intraspecific genetic variation within populations of I. brachytarsus is 0.4 ± 0.3% (range 0–1.5%, N = 64) for 16S mitochondrial gene sequences. Indirana brachytarsus is closely related to members of the I. beddomii group (Figs 1 and 2); from I. leithii by mean genetic divergence of 6.7 ± 0.6% (range 5.6–7.9%, N = 1280); from I. sarojamma by mean genetic divergence of 8.0 ± 0.4% (range 7.5–9.0%, N = 320); differs from I. tysoni by mean genetic divergence of 6.9 ± 0.2% (range 6.4–7.7%, N = 576); and from I. yadera by mean genetic divergence of 8.7 ± 0.3% (range 8.1–9.6%, N = 768) (S3 and S4 Tables). See I. beddomii and I. bhadrai for comparison with those species.
Distribution and natural history. Indirana brachytarsus is one of the most commonly occurring Indirana species in the Western Ghats states of Kerala and Tamil Nadu, but its distribution is restricted to the south of Palghat gap (Fig 3 and S1 Table). In our study, we found the northernmost occurrence of this species at Kaikatti (Nelliyampathy) in Palakkad district of Kerala, and at Andiparai shola (Valparai) in Coimbatore district of Tamil Nadu. In the present study, specimens were located mostly during the night (between 18:00–23:00 hours) either on the surface of wet rocks or on leaf litter near streams in moist deciduous to wet evergreen and semi-evergreen tropical forests, at elevations between 130–1508 m asl. For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
For morphological comparison, description of lectotype, secondary sexual characters, and list of specimens examined, see S1 File.
Indirana leithii (Boulenger 1888)
Matheran Leaping Frog [12]
(Figs 1–6; S2, S3 and S5 Figs; S1–S4 Tables; S1 File)
Original name and description. Rana leithii Boulenger 1888. Description of two new Indian species of Rana. Annals and Magazine of Natural History, Series 6, 1888(2): 506. Holotype. NHM 1947.2.28.17 (ex BMNH 1869.8.28.50), “single (female) specimen, presented by Dr. Leith”, by original designation. Type locality. “Matheran, Bombay”, India. Current status of specific name. Valid name, as Indirana leithii (Boulenger 1888).
Genetic divergence. Intraspecific genetic variation within populations of I. leithii is 0.7 ± 0.7% (range 0–1.7%, N = 20) for 16S mitochondrial gene sequences. I. leithii is closely related to members of the Indirana beddomii group (Figs 1 and 2); differs from I. sarojamma by mean genetic divergence of 6.2 ± 0.6% (range 5.5–7.3%, N = 100); from I. tysoni by mean genetic divergence of 6.1 ± 0.7% (range 5.2–7.1%, N = 180); and from I. yadera by mean genetic divergence of 7.0 ± 0.7% (range 6.1–8.2%, N = 240) (S3 and S4 Tables). See I. beddomii, I. bhadrai and I. brachytarsus for comparison with those species.
Distribution and natural history. The distribution of Indirana leithii is restricted to the state of Maharashtra, north of Goa gap (Fig 3 and S1 Table). In this study, we recorded this species at Bhimashankar (Pune district), Matheran (Raigad district), and Mahabaleshwar (Satara district). Specimens were located mostly during night searches (between 17:00 to 22:00 hours) either on wet laterite soil in a secondary forest patch (Matheran) or on wet leaf litter (Bhimashankar, Mahabaleshwar). For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
For morphological comparison and list of specimens examined, see S1 File.
Indirana sarojamma Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016
Sarojamma’s Leaping Frog [12]
(Figs 1–6; S2, S3 and S5 Figs; S1–S4 Tables; S1 File)
Original name and description. Indirana sarojamma Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016. Leaping frogs (Anura: Ranixalidae) of the Western Ghats of India: An integrated taxonomic review. Journal of Threatened Taxa, 2016(8): 9278–9280. Holotype. BNHS 5981, female, by original designation. Type locality. “Ponmudi Reserve Forest (8.736°N & 77.141°E, elevation 879m), Kerala, India”. Current status of specific name. Valid name, as Indirana sarojamma Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016.
Genetic divergence. Intraspecific genetic variation within populations of I. sarojamma was 0.2 ± 0.2% (range 0–0.4%, N = 5) for 16S mitochondrial gene sequences. I. sarojamma is closely related to members of the Indirana beddomii group (Figs 1 and 2); differs from I. tysoni by mean genetic divergence of 5.8 ± 0.2% (range 5.6–6.1%, N = 45); and from I. yadera by mean genetic divergence of 3.5 ± 0.1% (range 3.3–3.7%, N = 60) (S3 and S4 Tables). For comparison with I. beddomii, I. bhadrai, I. brachytarsus and I. leithii see ‘Genetic divergence’ of those species.
Distribution and natural history. Indirana sarojamma is currently known only from a small geographic region, south of Palghat gap in the Thiruvananthapuram district of Kerala state (Fig 3 and S1 Table). In the present study, we found this species on the surface of wet rocks close to primary forest at Chathankod–Bonnacaud (488 m asl), and under leaf litter on the floor of evergreen forest at Ponmudi (1014 m asl) in the Agasthyamala hills. Specimens were mostly located at night (between 18:00–21:00 hours). For details of the distribution records reported in this study see S1 Table.
For morphological comparison, variations, and list of specimens examined, see S1 File.
Indirana tysoni Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016
Tyson’s Leaping Frog [12]
(Figs 1–6; S2, S3 and S6 Figs; S1–S4 Tables; S1 File)
Original name and description. Indirana tysoni Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016. Leaping frogs (Anura: Ranixalidae) of the Western Ghats of India: An integrated taxonomic review. Journal of Threatened Taxa, 2016(8): 9258–9259. Holotype. BNHS 5979, male, by original designation. Type locality. “Ranipuram Vested Forest (12.419°N & 75.353°E, elevation 932m)”, Kerala, India. Current status of specific name. Valid name, as Indirana tysoni Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016.
Genetic divergence. Intraspecific genetic variation within populations of I. tysoni is zero (N = 9) for 16S mitochondrial gene sequences. I. tysoni is closely related to members of the I. beddomii group (Figs 1 and 2) and differs from I. yadera by mean genetic divergence of 5.9 ± 0.1% (range 5.6–6.1%, N = 108) (S3 and S4 Tables). For comparison with I. beddomii, I. bhadrai, I. brachytarsus, I. leithii and I. sarojamma see ‘Genetic divergence’ of those species.
Distribution and natural history. Indirana tysoni is currently known only from the Western Ghats states of Karnataka and Kerala, north of Palghat gap (Fig 3). In the present study, this species is reported from Karnataka: Chikmagalur district (Charmadi Ghats) and Kodagu district (Bhagamandala, Coorg, Madikeri and Thalakaveri). Specimens were located in secondary forest patches, mostly on surfaces of moss-covered rocks or moist leaf litter away from water-bodies during the late evening hours. During daytime (around 16:00 hours) they were found the under leaf litter. For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
For morphological comparison, secondary sexual characters, variations, and list of specimens examined, see S1 File.
Indirana yadera Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016
Yadera Leaping Frog [12]
(Figs 1–6; S2, S3 and S6 Figs; S1–S4 Tables; S1 File)
Original name and description. Indirana yadera Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016. Leaping frogs (Anura: Ranixalidae) of the Western Ghats of India: An integrated taxonomic review. Journal of Threatened Taxa, 2016(8): 9280–9282. Holotype. BNHS 5982, female, by original designation. Type locality. “Vathikudy, Idukki Wildlife Sanctuary (9.874°N & 77.076°E, elevation 797m)”, Kerala, India. Current status of specific name. Valid name, as Indirana yadera Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016.
Genetic divergence. Intraspecific genetic variation within populations of I. yadera is 0.4 ± 0.3% (range 0–1.1%, N = 12) for 16S mitochondrial gene sequences. I. yadera is closely related to members of the Indirana beddomii group (Figs 1 and 2; S3 and S4 Tables). For comparison with I. beddomii, I. bhadrai, I. brachytarsus, I. leithii, I. sarojamma and I. tysoni see ‘Genetic divergence’ of those species.
Distribution and natural history. Indirana yadera is currently known only from south of Palghat gap in the Western Ghats state of Kerala. In the present study, this species was collected from: Methooty in Idukki district, Neriamangalam in Ernakulam district, Pakuthipaalam (Nelliampathy) in Palakkad district, Kozhikana and Nilakkal in Pathanamthitta district, and Vazhachal in Thrissur district (Fig 3 and S1 Table). Specimens were mostly located during night searches (between 20:00–22:00 hours) on rock surfaces close to hill streams either in disturbed secondary forest patches (Methooty and Nelliyampathy) or undisturbed forests (Gavi and Vazhachal). For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
For morphological comparison, secondary sexual characters, variations, and list of specimens examined, see S1 File.
Indirana semipalmata group. Morphologically this group can be distinguished from I. beddomii group by the following suite of characters: small to medium-sized adult (male: SVL 23.0–43.0 mm, female: SVL 30.0–56.0 mm), first finger equal or nearly equal to second finger (Fig 4), tympanum well developed, large or nearly equal to horizontal diameter of eye (S2 Fig), fourth toe webbing extending up to or beyond the second subarticular tubercle on either side, except in I. semipalamata (Fig 5), and presence of prominent discontinuous longitudinal skin folds on dorsum.
Members included. Indirana chiravasi, I. duboisi, I. gundia, Indirana paramakri sp. nov., I. salelkari and I. semipalmata.
Indirana chiravasi Padhye, Modak and Dahanukar 2014
Amboli Leaping Frog [10]
(Figs 1–5 and 8; S2, S3 and S7 Figs; S1–S4 Tables; S1 File)
Fig 8. Indirana semipalmata group in life.
(A–C) I. chiravasi. (A) An adult male (not preserved), from Amboli. (B) An adult female (SDBDU 2012.2124), from Amboli. (C) An adult male (SDBDU 2012.2125), from Amboli. (D–F) I. duboisi. (D, E) An adult male (SDBDU 2014.2517), from Agumbe. (D) Dorsolateral view. (E) Frontal view. (F) An adult male (SDBDU 2011.1399), from Charmadi Ghats. (G–H) I. gundia. (G) An adult male (SDBDU 2008.433), from Kempholey. (H) An adult male (SDBDU 2002.1115), from Kempholey. (I) Indirana paramakri sp. nov., holotype, an adult female (ZSI/WGRC/V/A888), from Suganthagiri. (J) I. salelkari, an adult female (SDBDU 2011.1330), from Dandeli, dorsolateral view. (K–L) I. semipalmata. (K) An adult female (SDBDU 2015.3033), from Parambikulam. (L) An adult male (SDBDU 2015.3014), from Singappara.
Original name and description. Indirana chiravasi Padhye, Modak and Dahanukar 2014. Indirana chiravasi, a new species of leaping frog (Anura: Ranixalidae) from Western Ghats of India. Journal of Threatened Taxa, 2014(6): 6293–6312. Holotype. BNHS 5888, an adult male, by original designation. Type locality. “Amboli, Sindhudurg District, Maharashtra, India”. Current status of specific name. Valid name, as Indirana chiravasi Padhye, Modak and Dahanukar 2014.
Comment. We could not examine the type specimens of this species, as they were unavailable during our visits to the BNHS museum in September 2015 and February 2016.
Genetic divergence. Intraspecific genetic variation within populations of Indirana chiravasi is 0.1 ± 0.1% (range 0–0.4%, N = 17) for 16S mitochondrial gene sequences. I. chiravasi is closely related to members of the Indirana semipalmata group (Figs 1 and 2); differs from I. duboisi by mean genetic divergence of 3.0 ± 0.2% (range 2.7–3.7%, N = 323); from I. gundia by mean genetic divergence of 3.9 ± 0.3% (range 3.5–4.6%, N = 357); from I. paramakri by mean genetic divergence of 6.7 ± 0.5% (range 6.1–7.8%, N = 136); from I. salelkari by mean genetic divergence of 3.2 ± 0.4% (range 2.5–3.7%, N = 102); and from I. semipalmata by mean genetic divergence of 5.1 ± 0.4% (range 4.3–6.4%, N = 646) (S3 and S4 Tables).
Distribution and natural history. Indirana chiravasi is presently known to occur only in the northern Western Ghats, Maharashtra state. In the present study, this species is reported from Amboli (Sindhudurg district), Phansad WLS (Raigad district) and Koyna (Satara district) of Maharashtra (Fig 3 and S1 Table). For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
For morphological comparison and list of specimens examined, see S1 File.
Indirana duboisi Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016
Dubois’s Leaping Frog [12]
(Figs 1–5 and 8; S2, S3 and S7 Figs; S1–S4 Tables; S1 File)
Original name and description. Indirana duboisi Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016. Leaping frogs (Anura: Ranixalidae) of the Western Ghats of India: An integrated taxonomic review. Journal of Threatened Taxa, 2016(8): 9272–9274. Holotype. BNHS 5980, female, by original designation. Type locality. Kerekatte, Kudremukh National Park (13.322°N & 75.146°E, elevation 724m)”, Karnataka, India. Current status of specific name. Valid name, as Indirana duboisi Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016.
Genetic divergence. Intraspecific genetic variation within populations of Indirana duboisi is 0.5 ± 0.3% (range 0–1.3%, N = 19) for 16S mitochondrial gene sequences. I. duboisi is closely related to members of the Indirana semipalmata group (Figs 1 and 2); differs from I. gundia by mean genetic divergence of 3.2 ± 0.3% (range 2.4–3.9%, N = 399); from I. paramakri by mean genetic divergence of 5.4 ± 0.6% (range 4.5–7.0%, N = 152); from I. salelkari by mean genetic divergence of 2.6 ± 0.3% (range 2.1–3.3%, N = 114); and from I. semipalmata by mean genetic divergence of 4.7 ± 0.3% (range 4.2–5.6%, N = 722) (S3 and S4 Tables). See I. chiravasi for comparison with that species.
Distribution and natural history. Indirana duboisi is currently known only from the Western Ghats state of Karnataka, north of Palghat gap. In this study, we report the presence of this species in Chikmagalur district (Bygoor and Charmadi Ghats), Dakshin Kannada district (Gundia–Kempholey), Hassan district (Kempholey and Kottigehara), Shimoga district (Agumbe), and Uttara Kannada district (Castle rock and Kathlekan) (Fig 3 and S1 Table). Specimens were mostly found on forest floor, either on surface of wet rocks or on leaf litter, inside secondary forests. For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
For morphological comparison and list of specimens examined, see S1 File.
Indirana gundia (Dubois 1986)
Gundia Leaping Frog [12]
(Figs 1–5 and 8; S1–S3 and S8 Figs; S1–S4 Tables; S1 File)
Original name and description. Ranixalus gundia Dubois 1986. Diagnose préliminaire d’un nouveau genre de Ranoidea (Amphibiens, Anoures) du sud de l’Inde. Alytes, 1986(4): 114–118. Holotype. MNHNP 1985.633, female, by original designation. Type locality. “Gundia, forêt de Kemphole, à l'ouest de Sakleshpur, Karnataka, Inde”. Current status of specific name. Valid name, as Indirana gundia (Dubois 1986).
Genetic divergence. Intraspecific genetic variation within populations of Indirana gundia is 0.2 ± 0.2% (range 0–0.7%, N = 21) for 16S mitochondrial gene sequences. Indirana gundia is closely related to members of the Indirana semipalmata group (Figs 1 and 2); differs from I. paramakri by mean genetic divergence of 5.2 ± 0.5% (range 4.2–6.3%, N = 168); from I. salelkari by mean genetic divergence of 4.0 ± 0.3% (range 3.5–4.4%, N = 126); and from I. semipalmata by mean genetic divergence of 3.3 ± 0.2% (range 2.6–3.9%, N = 798) (S3 and S4 Tables). See I. chiravasi and I. duboisi for comparison with those species.
Distribution and natural history. Indirana gundia is currently known only from the Western Ghats states of Karnataka and Kerala, north of Palghat gap. In this study, we report the presence of this species at Gundia (Dakshin Kannada district), Kempholey (Hassan district), Monnangeri (Kodagu district) and Kudremukh (Udupi district) in Karnataka state; and Aralam (Kannur district) in Kerala state. The distribution of this species is restricted to the north of Palghat gap (Fig 3 and S1 Table). Animals were located both during day and night searches, mostly on forest floor covered with leaf litter or on rock surfaces near forest streams. For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
For morphological comparison, secondary sexual characters, and list of specimens examined, see S1 File.
Indirana paramakri sp. nov.
urn:lsid:zoobank.org:act:AFE5AB9D-9B57-4C01-A9A5-AD3D73FA3399
Rocky-terrain Leaping Frog
(Figs 1–5, 7 and 8; Table 1; S2 and S3 Figs; S1–S4 Tables)
Etymology. The species epithet ‘paramakri’ is derived from two Malayalam (the official language of Kerala state) words–para meaning ‘rock’ and makri for ‘frog’–referring to the predominant occurrence of Leaping frog species in rocky terrains. The species name is a noun standing in apposition to the generic name, and therefore invariable.
Holotype. ZSI/WGRC/V/A888, an adult female, from Suganthagiri (11.5386°N 76.0539°E, 852 m asl), Wayanad district, Kerala state, collected by SDB on 15 October 2005.
Paratypes. ZSI/WGRC/V/A889, an adult female, from Settukunnu (11.6172°N 75.9913°E, 823 m asl), Wayanad district, Kerala state, collected by SDB and SG on 5 June 2015; ZSI/WGRC/V/A890, an adult male, also from Settukunnu, Wayanad district, Kerala state, collected by SDB 20 July 2003.
Referred specimen. SDBDU 2005.3741, an adult male, from Suganthagiri (11.5386°N 76.0539°E, 852 m asl), Wayanad district, Kerala state, collected along with the holotype.
Comparison. Based on the overall morphology, Indirana paramakri sp. nov. could be confused with members of the Indirana semipalmata group. However, I. paramakri differs from I. chiravasi, I. duboisi, I. gundia, I. salelkari and I. semipalmata by its loreal region acute (vs. obtuse in all five species); specifically differs from I. duboisi, I. gundia, I. salelkari and I. semipalmata by its snout sub-ovoid in dorsal view (vs. nearly pointed in I. duboisi; rounded in I. gundia, I. salelkari and I. semipalmata); differs from I. chiravasi, I. duboisi, I. gundia and I. salelkari by its webbing between first, second and third toe well below the disc on the outside, I12/3–2+II13/4–23/4III2−–3IV3–1+V (vs. extending up to the disc in all four species) (Fig 5); specifically differs from I. semipalmata by its snout sub-ovoid in dorsal view (vs. rounded), fourth toe webbing extending up to the second subarticular tubercle on either side, I12/3–2+II13/4–23/4III2−–3IV3–1+V (vs. below, I2−–2+II2−–3−III2–31/4IV31/2–2V), and fifth toe webbing extending well above the first subarticular tubercle, I12/3–2+II13/4–23/4III2−–3IV3–1+V (vs. up to the first subarticular tubercle, I2−–2+II2−–3−III2–31/4IV31/2–2V) (Fig 5).
For better clarity, we compare this new species with all other currently known species in the genus. Indirana wayanadi differs from all the members of Indirana beddomii group by its webbing between first, second and third toe well below the disc on the outside, I12/3–2+II13/4–23/4III2−–3IV3–1+V (vs. extending up to the disc in all species) (Fig 5), and relatively smaller snout-vent size (except I. leithii), male SVL 27.4–30.1 mm, N = 2, female SVL 32.9–34.1 mm, N = 2 (vs. larger, I. beddomii male SVL 28.2–35.2 mm, N = 2, female SVL 44.8–55.5 mm, N = 5; I. bhadrai, male SVL 30.2 mm, N = 1, female SVL 38.7 mm, N = 1; I. brachytarsus, male SVL 30.6–34.3 mm, N = 3, female SVL 37.5–45.2 mm, N = 6; I. sarojamma, male SVL 34.5–38.2 mm, N = 2, female SVL 61.2 mm, N = 1; I. tysoni, male SVL 31.7–33.9 mm, N = 4, female SVL 51.3–52.8 mm, N = 2; I. yadera, male SVL 40.1–45.5 mm, N = 3, female SVL 58.6 mm, N = 1). More specifically differs from I. beddomii, I. leithii, I. sarojamma, I. tysoni and I. yadera by its first finger equal or nearly equal to second finger (vs. shorter in I. leithii, and longer in other four species) (Fig 4).
Furthermore, Indirana paramakri differs from members of the genus Sallywalkerana by its first finger equal or nearly equal to second finger (vs. shorter in all members) and fourth toe webbing extending up to the second subarticular tubercle on either side, I12/3–2+II13/4–23/4III2−–3IV3–1+V (vs. well below in all members) (Fig 5).
Genetic divergence. Intraspecific genetic variation within populations of Indirana paramakri sp. nov. is 0.6 ± 0.4% (range 0–1.3%, N = 8) for 16S mitochondrial gene sequences. Indirana paramakri is closely related to members of the Indirana semipalmata group (Figs 1 and 2); differs from I. salelkari by mean genetic divergence of 6.1 ± 0.5% (range 5.5–7.0%, N = 48); and from I. semipalmata by mean genetic divergence of 4.5 ± 0.5% (range 3.7–5.9%, N = 304) (S3 and S4 Tables). For comparison with I. chiravasi, I. duboisi and I. gundia see ‘Genetic divergence’ of those species.
Description of holotype (measurements in mm). Adult female (SVL 32.9); head small, longer than wide (HW 13.1, HL 13.8), flat above; snout sub-ovoid in dorsal view, rounded in lateral view, its length (SL 5.6) longer than horizontal diameter of eye (EL 4.1); loreal region acute and concave with rounded canthus rostralis; interorbital space flat, wider (IUE 3.4) than upper eyelid (UEW 2.7) and equal to internarial distance (IN 3.4); nostril closer to tip of snout (NS 1.8) than eye (EN 3.1); tympanum (TYD 3.1) 76% of eye diameter (EL 4.1). Forelimbs (FAL 6.6) shorter than hand length (HAL 7.8), finger length formula: IV<II<I<III, finger discs moderately wide compared to finger width FDI 0.8, FWI 0.5; FDII 0.8, FWII 0.6; FDIII 1.0, FWIII 0.7; FDIV1.0, FWIV 0.5). Thigh length (TL 16.7) shorter than shank (SHL 17.9), and longer than foot (FOL 16.2), toe discs wide compared to toe width (TDI 0.9, TWI 0.4; TDII 1.0, TWII 0.4; TDIII 1.2, TWIII 0.5; TDIV 1.3, TWIV 0.6; TDV 0.9, TWV 0.4), foot webbing: I12/3–2+II13/4–23/4III2−–3IV3–1+V.
Skin of snout and between eyes shagreened, upper eyelids shagreened to sparsely granular; posterior part of dorsum sparsely granular; dorsum with a few discontinuous longitudinal folds; lateral sides of head shagreened; anterior and posterior parts of flanks shagreened to sparsely granular; dorsal surface of forelimbs shagreened to finely granular; thigh, tibia and tarsus with weakly developed granular projections. Ventral surface of throat and chest smooth, abdomen and posterior parts of thigh prominently granular.
Colour of holotype. In life. Dorsum reddish-brown (Fig 8); a dark blackish-brown band extending from the nostril through the lower margin of eye, widening behind the eye and over the tympanum, and ending near the armpit on either sides of the head; tympanum blackish-brown, space between tympanum and eye dark blackish-brown; margins of upper and lower jaw with alternate dark brown and cream coloured cross-bars (Fig 8); forelimbs (including fingers) and hind limbs (including toes) reddish-brown with brown transverse bands; anterior and posterior parts of flanks light greyish-brown. Ventral surface light grey with a few scattered blackish-brown spots. In preservation. Dorsum greyish-brown with minute brown speckles; margins of upper and lower jaw with alternate dark brown and cream coloured cross-bars; a brown band extending from the nostril through the lower margin of eye, widening behind the eye and over the tympanum, and ending near the armpit on either sides of the head; tympanum brown; forelimbs and hindlimbs brown with dark brown transverse bands. Ventral surfaces light brown with a few scattered greyish-brown spot on throat and chest; ventral surface of limbs light greyish-brown, margins mottled with dark brown (Fig 7).
Secondary sexual characters. Male (ZSI/WGRC/V/A890), femoral glands present, nuptial pads present; female (ZSI/WGRC/V/A888), large pigmented eggs (diameter 0.5–0.8 mm, N = 20).
Variation. See Table 1 for morphometric data from two adult males and two adult females.
Distribution and natural history. Indirana paramakri is currently known only from two localities (Settukunnu and Suganthagiri), north of Palghat gap, in Wayanad district of the Western Ghats state of Kerala (Fig 3 and S1 Table). Animals were found in disturbed forest areas, either on surfaces of wet rocks near streams (Settukunnu) or under leaf litter and vegetation adjacent to a seasonal pond (Suganthagiri). Collections were made between 18:00–21:00 hours.
Indirana salelkari Modak, Dahanukar, Gosavi and Padhye 2015
Netravali Leaping Frog [11]
(Figs 1–5 and 8; S2, S3 and S8 Figs; S1–S4 Tables; S1 File)
Original name and description. Indirana salelkari Modak, Dahanukar, Gosavi and Padhye 2015. Indirana salelkari, a new species of leaping frog (Anura: Ranixalidae) from Western Ghats of Goa, India. Journal of Threatened Taxa, 2015(7): 7493–7509. Holotype. BNHS 5931, male, by original designation. Type locality. “Tanshikar Spice Farm in Neturlim (15.095°N & 74.211°E; elevation 78m), Sanguem Taluk, South Goa District, Goa, India”. Current status of specific name. Valid name, as Indirana salelkari Modak, Dahanukar, Gosavi and Padhye 2015.
Comment. We could not examine the type specimens of this species, as they were unavailable during our visits to the BNHS museum in September 2015 and February 2016.
Genetic divergence. Intraspecific genetic variation within populations of Indirana salelkari is 1.2 ± 0.9% (range 0–2.2%, N = 6) for 16S mitochondrial gene sequences. Indirana salelkari is closely related to members of the Indirana semipalmata group (Figs 1 and 2) and differs from I. semipalmata by mean genetic divergence of 5.4 ± 0.5% (range 4.3–6.3%, N = 228) (S3 and S4 Tables). See I. chiravasi, I. duboisi, I. gundia and I. paramakri for comparison with those species.
Distribution and natural history. Indirana salelkari is currently known only from the Western Ghats states of Goa and Karnataka, north of Palghat gap. The present study reports the occurrence of this species in Uttara Kannada district (Dandeli and Unchali falls) and Shimoga district (Jog falls) of Karnataka (Fig 3 and S1 Table). The presence of I. salelkari at Unchali falls and Jog falls was only confirmed genetically, as the specimen from Unchali falls was a sub-adult while only a tissue sample (without specimen) was collected from Jog falls. We observed this species on leaf litter or rock cuttings adjacent to streams inside secondary forests. For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
For morphological comparison and specimen examined see S1 File.
Indirana semipalmata (Boulenger 1882)
Brown Leaping Frog [58]
(Figs 1–5 and 8; S1–S3 and S8 Figs; S1–S4 Tables; S1 File)
Original name and description. Rana semipalmata Boulenger 1882. Catalogue of the Batrachia Salientia s. Ecaudata in the Collection of the British Museum. Second Edition. London: Taylor and Francis, 1882: 56–57. Lectotype. NHM 74.4.29.605 (ex BNHS 1947.2.29.50), designated by Dahanukar et al. [12], an adult female, from “Malabar”, figured in Boulenger 1882: Plate IV. Fig 3. Type locality. “Malabar”, India, according to original description. Current status of specific name. Valid name, as Indirana semipalmata (Boulenger 1882).
Comments. The original description [7] mentions two syntypes from Col. Beddome’s collection from “Malabar”. We examined the syntypes (a male and a female) at NHM, and found NHM 74.4.29.605 (ex BNHS 1947.2.29.50), an adult female, to be in agreement with the original description, with respect to snout-vent size (“From snout to vent 36 millim”), tympanum size (“tympanum distinct, about as large as the eye”) and webbing on foot (“toes half-webbed”). This specimen was also found to be in well-preserved condition (S8 Fig). Dahanukar et al. [12] designated NHM 74.4.29.605 (ex BNHS 1947.2.29.50) as the lectotype.
Genetic divergence. Intraspecific genetic variation within populations of Indirana semipalmata is 1.1 ± 0.7% (range 0–2.4%, N = 38) for 16S mitochondrial gene sequences. Indirana semipalmata is closely related to members of the Indirana semipalmata group (Figs 1 and 2; S3 and S4 Tables). See I. chiravasi, I. duboisi, I. gundia, I. paramakri and I. salelkari for comparison with those species.
Distribution and natural history. The distribution of Indirana semipalmata is restricted to the Western Ghats states of Kerala and Tamil Nadu. In the present study, this species is reported from Kerala: Ernakulam district (Neriamangalam), Idukki district (Kattapana, Kulamav, Pampadumpara and Periyar TR), Palakkad district (Nelliyampathi, Nenmara, Parambikulam TR and Siruvani), Pathanamthitta district (Gavi), and Thiruvananthapuram district (Chathankod–Bonnacaud and Kallar); Tamil Nadu: Top Slip, Karian Shola (Coimbatore district) (Fig 3 and S1 Table). Specimens were located during both day and night searches, and usually found on wet rocks close to streams or under leaf litter in primary and secondary forests. For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
For morphological comparison, description of lectotype, secondary sexual characters, variations, and list of specimens examined, see S1 File.
Genus. Sallywalkerana Dahanukar, Modak, Krutha, Nameer, Padhye and Molur 2016
Type species. Ixalus diplostictus Günther 1876 [= Sallywalkerana diplosticta (Günther 1876)]
Species included. Sallywalkerana diplosticta (Günther 1876), S. leptodactyla (Boulenger 1882), and S. phrynoderma (Boulenger 1882).
Distribution. The geographical range is restricted to south of Palghat gap in the Western Ghats. Distribution extends from Athirimala (Thiruvananthapuram district) to Eravikulam (Idukki district) in Kerala, and from Kakkachi (Tirunelveli district) to Valparai (Coimbatore district) in Tamil Nadu. For details of distribution records reported in the present study see Fig 3 and S1 Table.
Salient morphological characters. Male SVL 22.0–34.0 mm, female SVL 27.0–39.0 mm; pupil oval; presence of discontinuous dorsal skin folds or prominent skin warts; presence of various sized blotches on the ventral surface; vomerine teeth present; tongue emarginated posteriorly with lingual papillae; tympanum well-developed, smaller than horizontal diameter of eye (S2 Fig); first finger shorter than second finger (Fig 4); tips of fingers and toes with discs having distinct dorsoterminal grooves; interdigital webbing absent between fingers; webbing on foot reduced, just above the last subarticluar tubercles on toes II–IV (Fig 5).
Sallywalkerana diplosticta (Günther 1876)
Spotted Leaping Frog [58]
(Figs 1–5 and 9; S2, S3 and S9 Figs; S1–S4 Tables; S1 File)
Fig 9. Genus Sallywalkerana in life.
(A–D) S. diplosticta. (A, B) An adult male (SDBDU 2015.2956), from Pandipath. (A) Dorsolateral view. (B) Dorsal view. (C) An adult male (SDBDU 2003.40103), from Athirimala. (D) An adult male (SDBDU 2011.283), from Pandimotta. (E–G) S. leptodactyla. (E) An adult female (SDBDU 2013.911), from Mattupetti, dorsolateral view. (F) An adult female (SDBDU 2002.917), from Kodaikanal. (G) An adult female (SDBDU 2004.40336), from Valparai. (H, I) S. phrynoderma, an adult male (SDBDU 2002.1181), from Grass Hills. (H) Dorsolateral view. (I) Ventral view.
Original name and description. Ixalus diplostictus Günther 1876. Third report on collections of Indian reptiles obtained by the British Museum, Proceedings of the Zoological Society of London, 1876 “1875”: 574. Lectotype. NHM 1874.4.29.1412 (ex BMNH 1947.2.2.21), designated by Dahanukar et al. [12], adult female, from “Malabar”, figured in Günther 1876: LXIII. Fig C. Type locality. “Malabar”, India, according to original description. Current status of specific name. Valid name, as Sallywalkerana diplosticta (Günther 1876).
Comments. The original description [6] mentions four syntypes from “Malabar” based on Col. Beddome’s collection. We examined the three adult syntypes (one female and two males) available at NHM, and found NHM 1874.4.29.1412 (ex BMNH 1947.2.2.21), an adult female, to be in agreement with the original description, with respect to webbing between toes (“with a very short web”) and markings (“symmetrical black spots on the sides—one in front of the axil, another on the middle of the side of the trunk, a third above the loin”). This specimen was also found to be in relatively well-preserved condition (S9 Fig) and was designated as the lectotype by Dahanukar et al. [12].
Genetic divergence. Intraspecific genetic variation within populations of S. diplosticta is 1.1 ± 0.6% (range 0–1.7%, N = 9) for 16S mitochondrial gene sequences. S. diplosticta differs from S. leptodactyla by mean genetic divergence of 11.2 ± 0.4% (range 10.0–12.7%, N = 144); and from I. phrynoderma by mean genetic divergence of 12.2 ± 0.5% (range 11.5–12.7%, N = 18) (S3 and S4 Tables).
Distribution and natural history. Sallywalkerana diplosticta is currently known from a small geographical region in Agasthyamala hills, south of Palghat gap in the Western Ghats state of Kerala. In this study, we report this species from Kollam district (Pandimotta, Shendurney WLS) and Thiruvananthapuram district (Athirimala, Ponmudi and Pandipath) (Fig 3). Specimens were located during the night (between 18:00 to 21:00 hours) on wet rocks in primary forest at Athirimala, under vegetation near earthen cuttings at Pandipath, or on forest floor in evergreen forests at Ponmudi and Pandimotta. For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
See S1 File for morphological comparison, description of lectotype, secondary sexual characters, and list of specimens examined, and Fig 9 for colouration in life.
Sallywalkerana leptodactyla (Boulenger 1882)
Slender-toed Leaping Frog [12]
(Figs 1–5 and 9; S1–S3 and S9 Figs; S1–S4 Tables; S1 File)
Original name and description. Polypedates brevipalmatus Günther 1876 (Secondary homonym of Rana brevipalmata Peters, 1871). Third report on collections of Indian reptiles obtained by the British Museum, Proceedings of the Zoological Society of London, 1876 “1875”: 572–573. Lectotype. NHM 1874.4.29.593 (ex BMNH 1947.2.29.39), designated by Dahanukar et al. [12], adult female, from “Malabar”. Type locality. “Malabar”, India, according to original description. Current status of specific name. Valid name, as Sallywalkerana leptodactyla (Boulenger 1882).
Comments. The original description [6] mentions “several specimens” from “Malabar” and one specimen from “Anamallays”, based on Col. Beddome’s collection. We examined two adult syntypes (a female and a male) at NHM and found NHM 1874.4.29.593 (ex BMNH 1947.2.29.39), an adult female from “Malabar”, to be in agreement with the original description, with respect to webbing between toes (“toes long, with a very short web”), relative finger lengths (“Fingers without any web: the second rather longer than the first, and equal to the fourth, the third being the longest”), cross-band between the eyes (“a dark interocular cross-band”) and nearly similar SVL 35 mm (“Spec. B. 34 millim.”). This specimen was also found to be in relatively well-preserved condition (S9 Fig) and was designated as the lectotype by Dahanukar et al. [12].
Genetic divergence. Intraspecific genetic variation within populations of S. leptodactyla is 0.3 ± 0.3% (range 0–1.4%, N = 16) for 16S mitochondrial gene sequences. S. leptodactyla differs from S. phrynoderma by mean genetic divergence of 6.6 ± 0.3% (range 5.9–7.6%, N = 16) (S3 and S4 Tables). See S. diplosticta for comparison with that species.
Distribution and natural history. Sallywalkerana leptodactyla is currently known only from south of Palghat gap in the Western Ghats. In the present study, we report this species from Kerala: Idukki district (Eravikulam NP, Mattupetti and Munnar) and Thiruvananthapuram district (Ponkalapara) in Kerala; and Tamil Nadu: Coimbatore district (Grass hills and Valparai) and Dindigul district (Kodaikanal) (Fig 3 and S1 Table). Specimens were located during the night (between 16:00 to 20:00 hours), on surface of moss-covered rocks in open grasslands at Eravikulam NP, or under floor leaf litter in evergreen forest at Kodaikanal. For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
See S1 File for morphological comparison, description of lectotype, secondary sexual characters, variations, and list of specimens examined, and Fig 9 for colouration in life.
Sallywalkerana phrynoderma (Boulenger 1882)
Warty-skinned Leaping Frog [12]
(Figs 1–5 and 9; S1–S3 and S9 Figs; S1–S4 Tables; S1 File)
Original name and description. Rana phrynoderma Boulenger 1882. Catalogue of the Batrachia Salientia s. Ecaudata in the Collection of the British Museum. Second Edition. London: Taylor and Francis, 1882: 462. Lectotype. NHM 82.2.10.21 (ex BMNH 1947.2.3.8), designated by Dahanukar et al. [12], female, from “Anamallays”. Type locality. “Anamallays”, India, according to original description. Current status of specific name. Valid name, as Sallywalkerana phrynoderma (Boulenger 1882).
Comments. The original description [7] mentions “a-b. Hgr.” from Anamallays based on Col. Beddome’s collection. We examined the two adult syntypes (a female and a male) at NHM, and found NHM 82.2.1021 (ex BMNH 1947.2.3.8), “Anamallays” to be in agreement with the original description, with respect to its adult size (“35 millim”) and dorsal skin texture (“upper parts covered with warts of different sizes and short glandular folds”). Dahanukar et al. [12] designated this specimen as the lectotype.
Genetic divergence. Intraspecific genetic variation among populations of Sallywalkerana phrynoderma was zero (N = 2) for 16S mitochondrial gene sequences. S. phrynoderma is closely related to S. diplosticta and S. leptodactyla (Figs 1 and 2; S4 Table). See S. diplosticta and S. leptodactyla for comparison with these species.
Distribution and natural history. Sallywalkerana phrynoderma is known from a small geographical region, south of Palghat gap in the Western Ghats. In the present study we report this species from Grass Hills (Akkamalai shola) in Coimbatore district of Tamil Nadu state (Fig 3 and S1 Table). Specimens were located during the day (around 11:00 hours) on moist leaf litter in a forest patch adjacent to open grassland. For details of the distribution records reported in this study see S1 Table, and for other genetically confirmed records see S2 Table.
See S1 File for morphological comparison, secondary sexual characters, and list of specimens examined, and Fig 9 for colouration in life.
Discussion
While we formally describe two new species of Leaping frogs in the present work, our molecular data suggests that several other genetic lineages in family Ranixalidae (Fig 1) are likely to be complexes of cryptic species. For instance, a high degree of genetic differentiation is observed between various populations of Indirana beddomii (S3 Table). The distribution trend suggests genetic isolation between populations found north and south of the Palghat Gap, with up to 3.5% divergence for the mitochondrial 16S gene. A single DNA sequence of I. beddomii from Peruvannamuzhi (KX641761), situated north of Palghat gap, was found to nest with populations from south of Palghat gap, which could possibly be a locality error considering that other sequences (KX641759–60) from the same locality grouped with the northern populations (Fig 1). Two other species in our study were observed to have more than 2% intraspecific divergence (up to 2.2% for I. salelkari; up to 2.4% for I. semipalmata), which is comparable to the shallow interspecific genetic distances (minimum 2.1–2.7%) observed among some of the previously known members of the Indirana semipalmata group (e.g., minimum 2.1% between I. duboisi and I salelkari) (S4 Table). However, we found all these above-mentioned genetically divergent populations to be morphologically very similar to their closest relatives. Besides this, based on our preliminary genetic analysis one candidate species is suggested to be present in the genus Indirana (closely related to I. tysoni) and another in the genus Sallywalkerana (sister to S. leptodactyla). These populations could not be studied in the present work due to lack of voucher specimens or locality information for the DNA sequences found to be genetically divergent (JQ596673 and JQ596681–85, respectively), as well as sufficient samples in our collection. Nonetheless, the new and available genetic data clearly indicates the possible occurrence of undescribed cryptic ranixalid species. Considering the recent trends in description of numerous new anuran species from the Western Ghats, particularly in ancient and endemic lineages such as Micrixalidae [36] and Nyctibatrachidae [53], a high diversity of Leaping frogs is not unexpected but it is indicative that cryptic species remain undescribed even in recently and relatively well-studied groups. Detailed studies will be required to understand the observed intraspecific variations, particularly in Indirana beddomii, I. salelkari, I. semipalmata, I. tysoni and Sallywalkerana leptodactyla. Furthermore, genus Sallywalkerana was recognized largely based on reduced webbing, phylogenetic position and genetic divergence of >11% with all members of the genus Indirana [12]. According to our molecular analyses, high intraspecific genetic distances of >11% are even observed between Sallywalkerana diplosticta and populations of the other two Sallywalkerana species (with S. leptodactyla: mean 11.2%, range 10.0–12.7%; and with S. phrynoderma: mean 12.2%, range 11.5–12.7%) (S4 Table). Future integrated studies using multi-gene phylogenetic and phylogeographic analyses, bioacoustics as well as ecological data can provide better insights into the patterns of diversification and recognized taxonomic groupings in this ancient family.
Our findings also provide new insight into the distribution patterns of ranixalid species within the Western Ghats, while also reinforcing the role of elevational discontinuities (especially the 30 km wide Palghat gap) in determining distribution of anuran amphibians (e.g., [49, 52]). Members of the genus Sallywalkerana (S. diplosticta, S. leptodactyla and S. phrynoderma) are endemic to regions south of the Palghat gap, in the states of Kerala and Tamil Nadu. On the other hand, all the species in Indirana beddomii group and Indirana semipalmata group (except I. semipalmata) are restricted either to the north (Indirana beddomii group: I. beddomii, I. bhadrai, I. leithii and I. tysoni; Indirana semipalmata group: I. chiravasi, I. duboisi, I. gundia, I. paramakri and I. salelkari) or south (Indirana beddomii group: I. brachytarsus, I. sarojamma and I. yadera) of the Palghat gap (Fig 3). The exception–Indirana semipalmata is also largely restricted to the south of Palghat gap, but one population (from Siruvani) in our study was found north of this gap. Indirana leithii marks the northern most distribution limit of Leaping frogs, with its range restricted to the north of Goa gap, largely in the northern Western Ghats state of Maharashtra, as also discussed earlier [30]. In the present study, Indirana species were found between low-to mid elevations (66–1508 m asl) and many were usually observed close to human-altered landscapes in and around mixed deciduous (in Karnataka) to dry deciduous (Maharashtra) forests, which could make them prone to human-induced threats. On the other hand, Sallywalkerana species were found in mid-to high elevation forests (741–2310 m asl). Detailed natural history studies will be required to understand the various ecological adaptations of ranixalid members.
Ranixalid species have often been misidentified (e.g., [10, 11, 28, 29, 32, 58, 60–63]) due to their highly conserved external morphology resulting in taxonomic confusions and uncertainties. Dahanukar et al. [12] clarified the genetic identification of various previously available DNA sequences and the same is further confirmed in our study (Fig 1 and S2 Table). Besides this, we also provide additional distribution records for most of the previously known as well as all the recently described species, including many that were known only from their type localities. Hence, with the additional genetically and morphologically confirmed distribution records, descriptions of new species and new estimates of species diversity based on integrated evidence, our study provides an updated review of the family Ranixalidae. This will facilitate any future studies using integrated approaches to provide a better understanding of evolutionary relationships among ranixalid frogs.
Updated systematic revisions also provide vital information for conservation prioritization not only by enabling proper identification and range delineation of species, but also by facilitating conservation assessment of species (e.g., [64, 65]). According to the present conservation status of Leaping frogs [66], six species (genus Indirana: I. brachytarsus, I. gundia and I. leithii; genus Sallywalkerana: S. diplosticta, S. leptodactyla and S. phrynoderma) are already facing extinction threats, while others are unevaluated (genus Indirana: I. chiravasi, I. duboisi, I. salelkari, I. sarojamma, I. tysoni and I. yadera) (Fig 1C). With the availability of new information presented in this study, proper evaluation of all the previously known and newly described species will be facilitated. Since setting up of conservation priorities largely depend on the threat status of species [65], a reassessment of IUCN categorizations of all ranixalid species will be necessary for effective conservation of these relic frogs.
Through this work, we also draw attention to the taxonomic status of Indirana longicrus and I. tenuilingua, which have been considered as incertae sedis by Dahanukar et al. [12]. Longstanding taxonomic uncertainties regarding the status of these species [17] have existed largely due to unavailability of type specimens [31, 33] or new collections from the type locality. The new molecular and morphological data presented in our study can possibly yield different interpretations regarding the status of these two species. Though the original descriptions of I. longicrus and I. tenuilingua [13] discuss various ‘vague characters’ [12, 17], the type localities mentioned are not imprecise. Our genetic samples from Kempholey and vicinity are found to nest with the recently described I. duboisi [12] and I. gundia [9] (Fig 1 and S2 Table). Based on certain morphological characters (such as snout to vent length, tibium “longer than the thigh”, “arm equals the length of the snout”, tympanum “about half the diameter of the eye”) and the illustration accompanying the original description [13], our new collections from Kempholey and its vicinity could be assigned to I. longicrus, since this nominal taxon represents a member of the genus Indirana, as also previously noted by Bossuyt and Dubois [17]. However, in favour of maintaining taxonomic stability, we provisionally refrain from proposing new taxonomic actions to change the present status of I. longicrus and I. tenuilingua. At the same time, we find it important to point that available names should not be overlooked simply because of unavailability of type specimens and vague descriptions [17, 67, 68]. It would be prudent to acknowledge that taxonomy as a science has evolved drastically due to availability of modern tools and techniques (e.g., [69, 70]), which were unavailable for historical descriptions. Hence, it is advisable to refer to new collections from precisely known type localities for better insights and to use existing names, as much as possible (e.g., [36, 49, 53]), in order to allow ‘nomenclatural parsimony’ [71].
Supporting Information
(A, B) Indirana semipalmata, male (SDBDU 2015.3034) with femoral glands. (A) In life. (B) In preservation. (C) I. gundia, male (MNHN 1985.0633) with femoral glands (in preservation). (D) I. brachytarsus, male (SDBDU 2015.2931), without femoral glands (in life). (E) Sallywalkerana leptodactyla, male (SDBDU 2003.40336), without femoral glands (in life). (F) S. phrynoderma, female (SDBDU 2002.1 181), having a distinct bluish-black ventral surface with scattered grey color speckles (in life).
(PDF)
(A–G) Indirana beddomii group. (A) I. beddomii, male (SDBDU 2010.225) and female (SDBDU 2011.961). (B) I. bhadrai, female (ZSI/WGRC/V/A887). (C) I. brachytarsus, male (SDBDU 2015.2931) and female (SDBDU 2012.814). (D) I. leithii, male (SDBDU 2014. 2515) and female (SDBDU 2014.2514). (E) I. sarojamma, male (SDBDU 2002.334) and female (SDBDU 2002.516). (F) I. tysoni, male (SDBDU 2012.74) and female (SDBDU 2012.73). (G) I. yadera, male (SDBDU 2012.2744) and female (SDBDU 2015.3155). (H–M) Indirana semipalmata group. (H) I. chiravasi, male (SDBDU 2012.2125) and female (SDBDU 2015.3087). (I) I. duboisi, male (SDBDU 2003.1086) and female (SDBDU 2011.1399). (J) I. gundia, male (MNHN 1985.0633) and female (MNHN 1985.0621). (K) I. paramakri, male (SDBDU 2005.3741) and female (ZSI/WGRC/V/A888). (L) I. salelkari, female (SDBDU 2011.1330). (M) I. semipalmata, male (SDBDU 2015.3035) and female (SDBDU 2006.4773A). (N–P) Genus Sallywalkerana. (N) S. diplosticta, male (SDBDU 2003.40103) and female (SDBDU 2002.513). (O) S. leptodactyla, male (SDBDU 2004.40336) and female (SDBDU 2013.911). (P) S. phrynoderma, male (SDBDU 2002.1181).
(PDF)
(A–G) Indirana beddomii group. (A) I. beddomii, female (SDBDU 2011.961). (B) I. bhadrai, female (ZSI/WGRC/V/A887). (C) I. brachytarsus, female (SDBDU 2002.4091). (D) I. leithii, female (SDBDU 2002.2010). (E) I. sarojamma, female (SDBDU 2002.516). (F) I. tysoni, female (SDBDU 2012.73). (G) I. yadera, male (SDBDU 2012.2744). (H–M) Indirana semipalmata group. (H) I. chiravasi, female (SDBDU 2015.3087). (I) I. duboisi, male (SDBDU 2003.1086). (J) I. gundia, male (MNHN 1985.0633). (K) I. paramakri, female (ZSI/WGRC/V/A888). (L) I. salelkari, female (SDBDU 2011.1330). (M) I. semipalmata, female (SDBDU 2006.4773). (N–P) Genus Sallywalkerana. (N) S. diplosticta, female (SDBDU 2002.1249). (O) S. leptodactyla, female (SDBDU 2002.917). (P) S. phrynoderma, male (SDBDU 2002.1181).
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Lectotype of Polypedates beddomii (= Indirana beddomii), NHM 74.4.29.208 (ex BMNH 1947.2.27.72), female. (F–J) Lectotype of Polypedates brachytarsus (= Indirana brachytarsus), NHM 74.4.29.1307 (ex BMNH 1947.2.27.92), female.
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Holotype of Rana leithii (= Indirana leithii), NHM 69.8.28.50 (ex BMNH 1947.2.28.17), female. (F–J) Indirana sarojamma, SDBDU 2002.516, female.
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Indirana tysoni, SDBDU 2012.73, female. (F–J) Indirana yadera, SDBDU 2012.2744, male.
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Indirana chiravasi, SDBDU 2015.3087, female. (F–J) Indirana duboisi, SDBDU 2003.1086, male.
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Holotype of Ranixalus gundia (= Indirana gundia), MNHN 1985.0633, male. (F–J) Indirana salelkari, SDBDU 2011.1330, female. (K–O) Lectotype of Rana semipalmata (= Indirana semipalmata), NHM 74.4.29.605 (ex BMNH 1947.2.29.50), female.
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Lectotype of Ixalus diplostictus (= Sallywalkerana diplosticta), NHM 74.4.29.1412 (ex BMNH 1947.2.2.21), female. (F–J) Lectotype of Rana leptodactyla (= Sallywalkerana leptodactyla), NHM 74.4.29.593 (ex BMNH 1947.2.29.39), female. (K–O) Lectotype of Rana phrynoderma (= Sallywalkerana phrynoderma), NHM 82.2.10.21 (ex BMNH 1947.2.3.8), female.
(PDF)
(PDF)
Localities are arranged by State.
(PDF)
(PDF)
The table gives mean and standard deviation values over all pairwise comparisons among individuals or populations of a species. N is the number of individuals for each species. The original p-distances are shown in percentage.
(PDF)
The table gives mean and standard deviation values over all pairwise comparisons of individuals sequenced from the two taxa being compared. N is the number of pairwise comparisons. N1 and N2 represent number of individuals for Taxon 1 and Taxon 2, respectively. The original p-distances are shown in percentage.
(PDF)
Acknowledgments
The authors are grateful to the state forest departments of Maharashtra, Karnataka, Kerala and Tamil Nadu for providing study permits and field support; Barry Clark and Jeff Streicher (Natural History Musuem, London), Alain Dubois and Annemarie Ohler (Muséum National d’Histoire Naturelle, Paris), and Rahul Khot (Bombay Natural History Society, Mumbai) for providing access to specimens in their care; Matthias Stöck, Rafe Brown and an anonymous reviewer for helpful comments and suggestions which improved an earlier version of the manuscript; Franky Bossuyt for his support and encouragement to initiate this study during SDB’s PhD research at Vrije Universiteit Brussel; K Jayaram, K V Sreenivasan, Mallan Kani, Vijayan Kani and Sali Palode for invaluable assistance and logistical support in the field; Kailash Chandra and P M Sureshan (ZSI-WGRC) for help in accession of museum specimens; and past and present members of Systematics Lab for field and laboratory support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the following research grants to SDB: DU/DST ‘2009/868’ and ‘2014–16’ (Department of Science and Technology, Ministry of Science and Technology, Government of India); MoEF ‘23/3/2007-RE’ (Ministry of Environment, Forest and Climate Change, Government of India); CEPF ‘Project 55918/2009’ (Conservation International/Critical Ecosystem Partnership Fund, USA); and University of Delhi Research and Development Grants ‘2014–15’ and ‘2015–16’. SG was supported by fellowship from University of Delhi under the University Teaching Assistantship Scheme 2010/56562, and Senior Research Fellowship from Council for Scientific and Industrial Research, India (CSIR No. 9/45(1381)/2015-EMR-I).
References
- 1.Laurent RF. Sous-classe des lissamphibiens (Lissamphibia). Systématique. Traité de zoologie: anatomie, systématique, biologie. 1986;14: 594–797. [Google Scholar]
- 2.Dubois A. Miscellanea taxonomica batrachologica (I). Alytes. 1987;5(1–2): 7–95. [Google Scholar]
- 3.Dubois A. Notes sur la classification des Ranidae (Amphibiens anoures). Bull Mens Soc Linn Lyon. 1992;61: 305–352. [Google Scholar]
- 4.Blommers-Schlösser RMA. Systematic relationships of the Mantellinae Laurent 1946 (Anura, Ranoidea). Ethol Ecol Evol. 1993;5: 199–218. [Google Scholar]
- 5.Van Bocxlaer I, Roelants K, Biju SD, Nagaraju J, Bossuyt F. Late Cretaceous vicariance in Gondwanan amphibians. PLoS One. 2006;1: e74 10.1371/journal.pone.0000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ACLG. Third report on collections of Indian reptiles obtained by the British Museum. Proc Zool Soc. 1876;1875: 567–577. [Google Scholar]
- 7.Boulenger GA. Catalogue of the Batrachia Salientia s Ecaudata in the Collection of the British Museum. 2nd ed. London: Taylor and Francis; 1882. [Google Scholar]
- 8.Boulenger GA. Description of two new Indian species of Rana. J Nat Hist. 1888;2: 506–508. [Google Scholar]
- 9.Dubois A. Diagnose préliminaire d’un nouveau genre de Ranoidea (Amphibiens, Anoures) du sud de l’Inde. Alytes. 1986;4: 113–118. [Google Scholar]
- 10.Padhye AD, Modak N, Dahanukar N. Indirana chiravasi, a new species of leaping frog (Anura: Ranixalidae) from Western Ghats of India. J Threat Taxa. 2014;6: 6293–6312. [Google Scholar]
- 11.Modak N, Dahanukar N, Gosavi N, Padhye AD. Indirana salelkari, a new species of leaping frog (Anura: Ranixalidae) from Western Ghats of Goa, India. J Threat Taxa. 2015;7: 7493–7509. [Google Scholar]
- 12.Dahanukar N, Modak N, Krutha K, Nameer PO, Padhye AD, Molur S. Leaping frogs (Anura: Ranixalidae) of the Western Ghats of India: An integrated taxonomic review. J Threat Taxa. 2016;8: 9221–9288. [Google Scholar]
- 13.Rao CRN. On some new forms of Batrachia from S. India. Proc Indian Natl Sci Acad B Biol Sci. 1937;6: 387–427. [Google Scholar]
- 14.Boulenger GA. On the Papuan, Melanesian, and North Australian species of the genus Rana. J Nat Hist. 1918;1: 236–241. [Google Scholar]
- 15.Boulenger GA. A monograph of the South Asian, Papuan, Melanesian and Australian frogs of the genus Rana. Records of the Indian Museum. Calcutta, India: Director, Zoological Survey of India. 1920;20: 1–226. [Google Scholar]
- 16.Dubois A. Miscellanea nomenclatorica batrachologica (XV). Alytes. 1987;5: 175–176. [Google Scholar]
- 17.Bossuyt F, Dubois A. A review of the frog genus Philautus Gistel, 1848 (Amphibia, Anura, Ranidae, Rhacophorinae). Zeylanica. 2001;6: 1–112. [Google Scholar]
- 18.Vitt LJ, Caldwell JP. Herpetology: An Introductory Biology of Amphibians and Reptiles, 3th ed. Burlington, Massachusetts, USA: Academic Press; 2009. [Google Scholar]
- 19.Bossuyt F, Brown RM, Hillis DM, Cannatella DC, Milinkovitch MC. Phylogeny and biogeography of a cosmopolitan frog radiation: Late Cretaceous diversification resulted in continent-scale endemism in the family Ranidae. Syst Biol. 2006;55: 579–594. 10.1080/10635150600812551 [DOI] [PubMed] [Google Scholar]
- 20.Vences M, Wanke S, Odierna G, Kosuch J, Veith M. Molecular and karyological data on the South Asian ranid genera Indirana, Nyctibatrachus and Nannophrys (Anura: Ranidae). Hamadryad. 2000;25: 75–82. [Google Scholar]
- 21.Bossuyt F, Milinkovitch MC. Amphibians as indicators of Early Tertiary “out-of-India” dispersal of vertebrates. Science. 2001;292: 93–95. 10.1126/science.1058875 [DOI] [PubMed] [Google Scholar]
- 22.Vences M, Glaw F. When molecules claim for taxonomic changes: new proposals on the classification of Old World tree frogs. Spixiana. 2001;24: 85–92. [Google Scholar]
- 23.Roelants K, Jiang J, Bossuyt F. Endemic ranid (Amphibia: Anura) genera in southern mountain ranges of the Indian subcontinent represent ancient frog lineages: evidence from molecular data. Mol Phylogenet Evol. 2004; 31:730–740. 10.1016/j.ympev.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 24.Van der Meijden A, Vences M, Hoegg S, Meyer A. A previously unrecognized radiation of ranid frogs in southern Africa revealed by nuclear and mitochondrial DNA sequences. Mol Phylogenet Evol. 2005;37: 674–685. 10.1016/j.ympev.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 25.Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, et al. The amphibian tree of life. Bull Am Mus Nat Hist. 2006;297: 1–370. 10.1206/0003-0090(2006)297[0001:TATOL]2.0.CO;2 [DOI] [Google Scholar]
- 26.Wiens JJ, Sukumaran J, Pyron RA, Brown RM. Evolutionary and biogeographic origins of high tropical diversity in Old World frogs (Ranidae). Evolution. 2009;63: 1217–1231. 10.1111/j.1558-5646.2009.00610.x [DOI] [PubMed] [Google Scholar]
- 27.Pyron RA, Wiens JJ. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phylogenet Evol. 2011;61: 543–583. 10.1016/j.ympev.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 28.Nair A, Gopalan SV, George S, Kumar KS, Teacher AGF, Merilä J. High cryptic diversity of endemic Indirana frogs in the Western Ghats biodiversity hotspot. Anim Conserv. 2012;15(5): 489–98. [Google Scholar]
- 29.Nair A, Gopalan SV, George S, Kumar KS, Shikano T, Merilä J. Genetic variation and differentiation in Indirana beddomii frogs endemic to the Western Ghats biodiversity hotspot. Conserv Genet. 2012;13: 1459–1467. [Google Scholar]
- 30.Modak N, Padhye AD, Dahanukar N. Delimiting the distribution range of Indirana leithii (Boulenger, 1888) (Anura: Ranixalidae), an endemic threatened anuran of the Western Ghats, based on molecular and morphological analysis. Zootaxa. 2014;3796: 62–80. [DOI] [PubMed] [Google Scholar]
- 31.Biju SD. A synopsis to the frog fauna of the Western Ghats, India. Indian Society for Conservation Biology, Occasional publication. 2001;1: 1–24. [Google Scholar]
- 32.Nair A, Gopalan SV, George S, Kumar KS, Teacher AGF, Merilä J. Endemic Indirana frogs of the Western Ghats biodiversity hotspot. Ann Zool Fennici. 2012;49: 257–286. [Google Scholar]
- 33.Dubois A. Note préliminaire sur le groupe de Rana limnocharis Gravenhorst, 1829 (Amphibiens, Anoures). Alytes. 1984;3: 143–159. [Google Scholar]
- 34.Myers N, Mittermier RA, Mittermier CG, Fonescaand da GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403: 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- 35.Biju SD, Bossuyt F. New frog family from India reveals an ancient biogeographical link with the Seychelles. Nature. 2003; 425(6959):711–714. 10.1038/nature02019 [DOI] [PubMed] [Google Scholar]
- 36.Biju SD, Garg S, Gururaja KV, Shouche YS, Walujkar SA. DNA barcoding reveals unprecedented diversity in dancing frogs of India (Micrixalidae, Micrixalus): a taxonomic revision with description of 14 new species. Ceylon J Sci Biol Sci. 2014; 43:1–87. [Google Scholar]
- 37.Frost DR. Amphibian Species of the World: an Online Reference, Version 6.0. Electronic Database. 2016. Available: http://researchamnhorg/vz/herpetology/amphibia/.
- 38.AmphibiaWeb. Information on amphibian biology and conservation (web application). 2016. Berkeley, California: AmphibiaWeb. Available: http://amphibiaweb.org.
- 39.Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994;87(6): 651–701. 10.1093/aesa/87.6.651 [DOI] [Google Scholar]
- 40.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28: 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (* and other methods), Version 4.0b10. Sunderland, Massachusetts: Sinauer Association Inc; 2002.
- 42.Meyer CP, Paulay G. DNA barcoding: error rates based on comprehensive sampling. PLoS Biol. 2005;3: 2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14(9): 817–818. [DOI] [PubMed] [Google Scholar]
- 44.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21): 2688–2990. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 45.Stamatakis A. A rapid bootstrap algorithm for the RAxML Web Servers. Syst. Biol. 2008;57(5): 758–71. 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- 46.Silvestro D, Michalak I. raxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 2012;12(4): 335–337. [Google Scholar]
- 47.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12): 1572–1574. [DOI] [PubMed] [Google Scholar]
- 48.Biju SD, Van Bocxlaer I, Giri VB, Loader SP, Bossuyt F. Two new endemic genera and a new species of toad (Anura: Bufonidae) from the Western Ghats of India. BMC Res Notes. 2009;2(241): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biju SD, Garg S, Mahony S, Wijayathilaka N, Senevirathne G, Meegaskumbura M. DNA barcoding, phylogeny and systematics of Golden-backed frogs (Hylarana, Ranidae) of the Western Ghats-Sri Lanka biodiversity hotspot, with the description of seven new species. Contrib Zool. 2014; 83(4): 269–335. http://www.contributionstozoology.nl/vol83/nr04/a04 [Google Scholar]
- 50.Savage JM, Heyer WR. Variation and distribution in the tree-frog genus Phyllomedusa in Costa Rica, Central America: With 6 figures. Stud Neotrop Fauna Environ. 1967; 5(2): 111–131. [Google Scholar]
- 51.Myers CW, Duellman WE. A New Species of Hyla from Cerro Colorado, and other Tree frog records and geographical notes from Western Panama. Am Mus Novit. 1982; 2752: 1–32. [Google Scholar]
- 52.Van Bocxlaer I, Biju SD, Willaert B, Giri VB, Shouche YS, Bossuyt F. Mountain-associated clade endemism in an ancient frog family (Nyctibatrachidae) on the Indian subcontinent. Mol Phylogenet Evol. 2012;62: 839–847. 10.1016/j.ympev.2011.11.027 [DOI] [PubMed] [Google Scholar]
- 53.Biju SD, Van Bocxlaer I, Mahony S, Dinesh KP, Radhakrishnan C, Zachariah A, et al. A taxonomic review of the Night Frog genus Nyctibatrachus Boulenger, 1882 in the Western Ghats, India (Anura: Nyctibatrachidae) with description of twelve new species. Zootaxa. 2011;3029: 1–96. [Google Scholar]
- 54.Daniel JC, Shull EM. 1964. A list of the reptiles and amphibians of Surat Dangs, South Gujarat. J Bombay Nat Hist Soc. 1964;60: 737–743. [Google Scholar]
- 55.Srinivasulu C, Das I. The herpetofauna of Nallamala hills, Eastern Ghats, India: annotated checklist, with remarks on nomenclature, taxonomy, habitat use, adaptive types and biogeography. Asiat Herpetol Res. 2008;11: 110–131. [Google Scholar]
- 56.Dutta SK. Amphibians of India: updated species list with distribution record. Hamadryad. 1992;17: 1–3. [Google Scholar]
- 57.Chandra K, Gajbe PU. An inventory of herpetofauna of Madhya Pradesh and Chhattisgarh. Zoos Print J. 2005;20: 1812–1819. [Google Scholar]
- 58.Daniels RJR. Amphibians of Peninsular India. Hyderabad: Universities Press (India) Private Ltd; 2005. [Google Scholar]
- 59.Inger RF, Shaffer HB, Koshy M, Bakde R. A report on a collection of amphibians and reptiles from Ponmudi, Kerala, South India. J Bombay Nat Hist Soc. 1984;81: 406–427. [Google Scholar]
- 60.Daniel JC, Sekar AG. Field guide to the amphibians of Western India. J Bombay Nat Hist Soc. 1989;86: 194–202. [Google Scholar]
- 61.Dutta SK. Amphibians of India and Sri Lanka (Checklist and Bibliography) Bhubaneswar: Odyssey Publishing House; 1997. [Google Scholar]
- 62.Chanda SK. Handbook of Indian amphibians. Kolkata: Zoological Survey of India; 2002. [Google Scholar]
- 63.Reddy AH, Gururaja KV, Ravichandran MS, Krishnamurthy SV. Range extension of Ansonia ornata (Gunther, 1875) and Indirana brachytarsus (Gunther, 1875). Hamadryad. 2001;26: 358–359. [Google Scholar]
- 64.Vieites DR, Wollenberg KC, Andreone F, Köhler J, Glaw F, Vences M. Vast underestimation of Madagascar's biodiversity evidenced by an integrative amphibian inventory. Proc Natl Acad Sci U S A. 2009;106(20): 8267–72. 10.1073/pnas.0810821106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glaw F, Köhler J, De la Riva I, Vieites DR, Vences M. Integrative taxonomy of Malagasy treefrogs: combination of molecular genetics, bioacoustics and comparative morphology reveals twelve additional species of Boophis. Zootaxa. 2010;2383(1): 82. [Google Scholar]
- 66.IUCN 2015. IUCN Red List of Threatened Species. Version 2015–3. Available: http://www.iucnredlist.org.
- 67.Dubois A, Ohler A. Frogs of the subgenus Pelophylax (Amphibia, Anura, genus Rana): a catalogue of available and valid scientific names, with comments on name-bearing types, complete synonymies, proposed common names, and maps showing all type localities. Zool Pol. 1995 “1994. ”;39(3–4): 139–204. [Google Scholar]
- 68.Dubois A, Ohler A. Early scientific names of Amphibia Anura. I. Introduction. Bull Mus Natn Hist Nat. 1997 “1996. ”;18(3–4): 297–320. [Google Scholar]
- 69.Hanken J. Why are there so many new amphibian species when amphibians are declining? Trends Ecol Evol. 1999;14(1): 7–8. [DOI] [PubMed] [Google Scholar]
- 70.Köhler J, Vieites DR, Bonett RM, García FH, Glaw F, Steinke D, et al. New amphibians and global conservation: a boost in species discoveries in a highly endangered vertebrate group. BioScience. 2005;55(8): 693–696. [Google Scholar]
- 71.Dubois A. New proposals for naming lower-ranked taxa within the frame of the International Code of Zoological Nomenclature. C R Biol. 2006;329(10): 823–840. 10.1016/j.crvi.2006.07.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A, B) Indirana semipalmata, male (SDBDU 2015.3034) with femoral glands. (A) In life. (B) In preservation. (C) I. gundia, male (MNHN 1985.0633) with femoral glands (in preservation). (D) I. brachytarsus, male (SDBDU 2015.2931), without femoral glands (in life). (E) Sallywalkerana leptodactyla, male (SDBDU 2003.40336), without femoral glands (in life). (F) S. phrynoderma, female (SDBDU 2002.1 181), having a distinct bluish-black ventral surface with scattered grey color speckles (in life).
(PDF)
(A–G) Indirana beddomii group. (A) I. beddomii, male (SDBDU 2010.225) and female (SDBDU 2011.961). (B) I. bhadrai, female (ZSI/WGRC/V/A887). (C) I. brachytarsus, male (SDBDU 2015.2931) and female (SDBDU 2012.814). (D) I. leithii, male (SDBDU 2014. 2515) and female (SDBDU 2014.2514). (E) I. sarojamma, male (SDBDU 2002.334) and female (SDBDU 2002.516). (F) I. tysoni, male (SDBDU 2012.74) and female (SDBDU 2012.73). (G) I. yadera, male (SDBDU 2012.2744) and female (SDBDU 2015.3155). (H–M) Indirana semipalmata group. (H) I. chiravasi, male (SDBDU 2012.2125) and female (SDBDU 2015.3087). (I) I. duboisi, male (SDBDU 2003.1086) and female (SDBDU 2011.1399). (J) I. gundia, male (MNHN 1985.0633) and female (MNHN 1985.0621). (K) I. paramakri, male (SDBDU 2005.3741) and female (ZSI/WGRC/V/A888). (L) I. salelkari, female (SDBDU 2011.1330). (M) I. semipalmata, male (SDBDU 2015.3035) and female (SDBDU 2006.4773A). (N–P) Genus Sallywalkerana. (N) S. diplosticta, male (SDBDU 2003.40103) and female (SDBDU 2002.513). (O) S. leptodactyla, male (SDBDU 2004.40336) and female (SDBDU 2013.911). (P) S. phrynoderma, male (SDBDU 2002.1181).
(PDF)
(A–G) Indirana beddomii group. (A) I. beddomii, female (SDBDU 2011.961). (B) I. bhadrai, female (ZSI/WGRC/V/A887). (C) I. brachytarsus, female (SDBDU 2002.4091). (D) I. leithii, female (SDBDU 2002.2010). (E) I. sarojamma, female (SDBDU 2002.516). (F) I. tysoni, female (SDBDU 2012.73). (G) I. yadera, male (SDBDU 2012.2744). (H–M) Indirana semipalmata group. (H) I. chiravasi, female (SDBDU 2015.3087). (I) I. duboisi, male (SDBDU 2003.1086). (J) I. gundia, male (MNHN 1985.0633). (K) I. paramakri, female (ZSI/WGRC/V/A888). (L) I. salelkari, female (SDBDU 2011.1330). (M) I. semipalmata, female (SDBDU 2006.4773). (N–P) Genus Sallywalkerana. (N) S. diplosticta, female (SDBDU 2002.1249). (O) S. leptodactyla, female (SDBDU 2002.917). (P) S. phrynoderma, male (SDBDU 2002.1181).
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Lectotype of Polypedates beddomii (= Indirana beddomii), NHM 74.4.29.208 (ex BMNH 1947.2.27.72), female. (F–J) Lectotype of Polypedates brachytarsus (= Indirana brachytarsus), NHM 74.4.29.1307 (ex BMNH 1947.2.27.92), female.
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Holotype of Rana leithii (= Indirana leithii), NHM 69.8.28.50 (ex BMNH 1947.2.28.17), female. (F–J) Indirana sarojamma, SDBDU 2002.516, female.
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Indirana tysoni, SDBDU 2012.73, female. (F–J) Indirana yadera, SDBDU 2012.2744, male.
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Indirana chiravasi, SDBDU 2015.3087, female. (F–J) Indirana duboisi, SDBDU 2003.1086, male.
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Holotype of Ranixalus gundia (= Indirana gundia), MNHN 1985.0633, male. (F–J) Indirana salelkari, SDBDU 2011.1330, female. (K–O) Lectotype of Rana semipalmata (= Indirana semipalmata), NHM 74.4.29.605 (ex BMNH 1947.2.29.50), female.
(PDF)
From left to right: Dorsal view, ventral view, lateral view of head, ventral view of hand, ventral view of foot. (A–E) Lectotype of Ixalus diplostictus (= Sallywalkerana diplosticta), NHM 74.4.29.1412 (ex BMNH 1947.2.2.21), female. (F–J) Lectotype of Rana leptodactyla (= Sallywalkerana leptodactyla), NHM 74.4.29.593 (ex BMNH 1947.2.29.39), female. (K–O) Lectotype of Rana phrynoderma (= Sallywalkerana phrynoderma), NHM 82.2.10.21 (ex BMNH 1947.2.3.8), female.
(PDF)
(PDF)
Localities are arranged by State.
(PDF)
(PDF)
The table gives mean and standard deviation values over all pairwise comparisons among individuals or populations of a species. N is the number of individuals for each species. The original p-distances are shown in percentage.
(PDF)
The table gives mean and standard deviation values over all pairwise comparisons of individuals sequenced from the two taxa being compared. N is the number of pairwise comparisons. N1 and N2 represent number of individuals for Taxon 1 and Taxon 2, respectively. The original p-distances are shown in percentage.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.