Table 3.
Coupling of various glycosyl acceptors to C(2)-N-benzylidene substituted glucosamine donor 5.
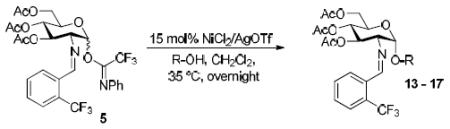
| Entry | Acceptors | Products | Yieldd (α:βe) |
|---|---|---|---|
| 1a |
|
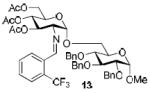
|
85%, 15:1 |
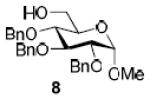
| 2b |
|
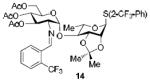
|
93%, α-only |
| 3a |
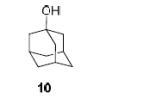
|
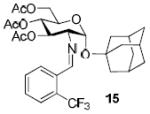
|
90%, 11:1 |
| 4a |
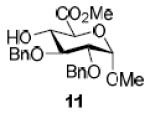
|
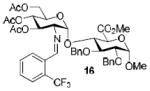
|
76%, α-only 71%, α-onlyf |
| 5c |
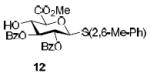
|
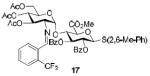
|
58%, α-only |
Donor/Acceptor = 1:1.2.
Donor/Acceptor = 1:2.
Donor/Acceptor = 2:1.
Isolated by chromatography.
Calculated based on 1H NMR.
Conducted with 15 mol % commercially available Ni(OTf)2 for 16 h.
