Abstract
Early screens in yeast for mutations exhibiting sensitivity to DNA damage identified nuclear pore components, but their role in DNA repair was not well understood. Over the last decade, studies have revealed that several types of persistent DNA lesions relocate to either the nuclear pore complex (NPC) or nuclear envelope (NE). Of these two sites, the nuclear pore appears to be crucial for DNA repair of persistent double-strand breaks, eroded telomeres and sites of fork collapse at expanded CAG repeats. Using a combination of cell biological imaging techniques and yeast genetic assays for DNA repair, researchers have begun to understand both the how and why of lesion relocation to the NPC. Here we review the types of lesions that relocate to the NPC, mediators of relocation and the functional consequences of relocation understood to date. The emerging theme is that relocation to the NPC regulates recombination to influence repair pathway choice and provide a rescue mechanism for lesions or DNA structures that are resistant to repair.
Keywords: nuclear pore complex, recombination, DNA repair, collapsed replication fork, persistent DSB, eroded telomere
The authors review how relocation of DNA lesions to the nuclear pore complex influences repair pathway choice and promotes genome stability.
ABBREVIATIONS
- DSB:
double strand break
- HR:
homologous recombination
- BIR:
break-induced replication
- SSA:
single strand annealing
- MMEJ:
microhomology-mediated end joining
- NHEJ:
non-homologous end joining
- uSCR:
unequal sister chromatid recombination
- GCR:
gross chromosomal rearrangement
- ChIP:
Chromatin Immunoprecipitation
- HO:
homothallic endonuclease (or cut site)
- HU:
hydroxyurea
- MMS:
methylmethanesulfonate
- NPC:
nuclear pore complex
- NE:
nuclear envelope
INTRODUCTION
DNA repair must occur in the context of a crowded nucleus. For homologous recombination (HR), the additional challenge exists of finding the right homologous template for repair. This is facilitated by robust search mechanisms, including proteins such as Rad51 that mediate synapsis (Symington, Rothstein and Lisby 2014) and a regulated increase in mobility of the broken chromosome (Dion et al.2012; Mine-Hattab and Rothstein 2012; Dion and Gasser 2013). In late S or G2 phases, once a sister chromatid is available, it is the preferred template for repair, which is facilitated by proximity maintained by sister chromatid cohesion (Nasmyth and Haering 2009). Spontaneous damage that occurs in S phase is often repaired very quickly, in a manner of minutes (Lisby, Rothstein and Mortensen 2001). However, some types of damage appear to be more difficult to repair, and can persist, sometimes for hours, causing G2 arrest. In the last decade, it has been appreciated that some of these types of persistent lesions relocate to the nuclear periphery, and this relocation appears to play an important role in rescuing the repair process and facilitating its completion (see Lisby et al.2010; Nagai, Heun and Gasser 2010; Nagai, Davoodi and Gasser 2011; Geli and Lisby 2015 for other recent reviews). In this review, we will provide a brief summary of the current state of the field, focusing on the types of lesions known to relocate to the nuclear pore in Saccharomyces cerevisiae, and the possible roles of this event in facilitating or regulating DNA repair and fork restart.
STRUCTURE OF THE YEAST NUCLEAR PERIPHERY
The nuclear periphery is the boundary between the nucleus and cytoplasm, which is composed of a nuclear envelope (NE) and many nuclear pores. In yeast, the NE is a bilayer membrane composed of inner and outer nuclear membranes. The yeast inner nuclear membrane contains at least 10 proteins, including Mps3, an essential Sad1-UNC-84 (SUN) domain protein (Lusk, Blobel and King 2007). Mps3 is required for duplication of the spindle pole body and telomere peripheral tethering (Jaspersen, Giddings and Winey 2002; Nishikawa et al.2003; Antoniacci, Kenna and Skibbens 2007; Bupp et al.2007). The non-essential N-terminal domain of Mps3 is not required for spindle pole body duplication and Mps3 integration to the envelope, but it is required for telomere and double-strand break (DSB) positioning (Bupp et al.2007; Oza et al.2009). In mammalian cells, there is also a protein network of intermediate filaments lining the inner nuclear membrane called lamins, which are important in DNA replication, repair and genome stability maintenance (Singh et al.2013; Gruenbaum and Foisner 2015).
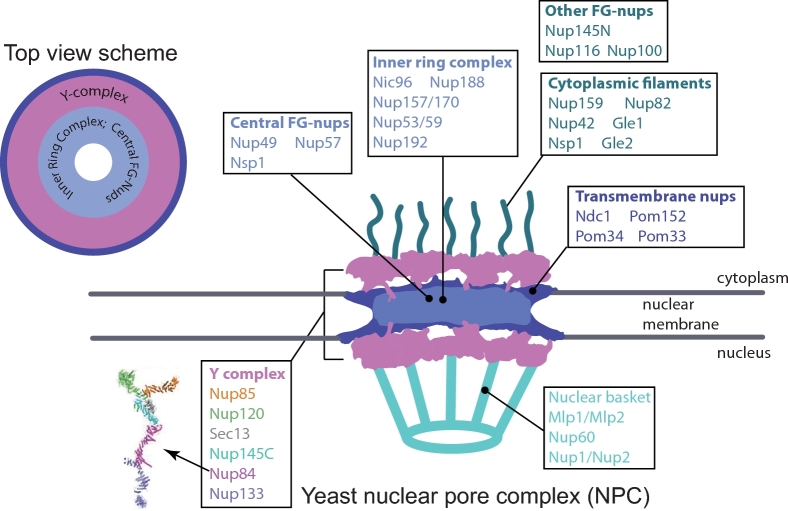
Penetrating the NE are the nuclear pores, about 100–200 per cell in yeast, which provide a conduit between the nuclear and cytoplasmic compartments. The nuclear pore complex (NPC) is made up of at least 30 nucleoporins (usually named ‘nups’), and different groups of porins are further assembled into subcomplexes, which make up the basic building blocks of a nuclear pore (Schwartz 2016). The nuclear pore structure is highly conserved between yeast and more complex eukaryotic organisms. The largest nuclear pore subcomplex is the Y-shaped Nup84 complex (mammalian Nup107) (Fig. 1). It is a multiprotein complex with six core conserved members, and is the main component of the ring structure present on both nuclear and cytoplasmic sides of the pore (Fig. 1) (Schwartz 2016). There are several other subcomplexes located primarily internal to the ring structure formed by the Y complex (Fig. 1). The nuclear basket interacts with the pore ring and protrudes into the nucleoplasm; its components, Mlp1, Mlp2, Nup60, Nup1 and Nup2, are important in RNA export, gene gating, transcriptional regulation and DNA repair (Palancade et al.2007; Sood and Brickner 2014). The Y complex is also important for DNA repair, as mutation of Nup84, Nup120 or Nup133 causes hypersensitivity to DNA damaging agents, accumulation of spontaneous Rad52 foci and synthetic lethality with mutations that impair HR (Bennett et al.2001; Loeillet et al.2005; Palancade et al.2007; Nagai et al.2008). Nuclear pores have a highly conserved structure and function between human and yeast cells (Mekhail and Moazed 2010).
Figure 1.
Saccharomyces cerevisiae NPC structure. The diagram is drawn based on Schwartz (2016); the composite high-resolution Y-shape complex structure is from Kelley et al. (2015). FG-nups are nucleoporins containing repetitive phenylalanine (F) and glycine (G) amino acid sequences.
RELOCATION OF DAMAGED DNA WITHIN THE NUCLEUS
Relocation of damaged DNA within the nucleus has been observed for several circumstances. Early on, it was discovered that DSBs within the ribosomal DNA (rDNA) relocate to a position outside the nucleolus during repair, and failure to do so leads to loss of rDNA repeats (Torres-Rosell et al.2007). Similarly, breaks in heterochromatic DNA move outside the locus to be repaired in Drosophila and mammalian cells (Chiolo et al.2011; Jakob et al.2011; Ryu et al.2015; Tsouroula et al.2016). Induction of a DSB in a yeast chromosome by the homothallic (HO) endonuclease does not lead to relocation. However, if the homologous donor sequences normally used for repair are removed or even moved to another chromosome, the broken chromosome relocates to the nuclear periphery (Nagai et al.2008; Kalocsay, Hiller and Jentsch 2009; Oza et al.2009). Similarly, a break that can heal by single strand annealing (SSA) with only 5 kb of resection does not relocate, but one that requires 30 kb of resection does (Oza et al.2009). These persistent or ‘difficult to repair’ DSBs relocate either to the nuclear pore via interaction with the Nup84 subcomplex or to the INM via interaction with Mps3. Recently, it was shown that the site of DSB relocation can depend on the cell cycle phase: DSB interaction with the Mps3 protein is restricted to S and G2 phases, whereas NPC interactions can occur in any cell cycle phase (Horigome et al.2016). The interaction site can also depend on DNA structure, as eroded telomeres in telomerase-deficient cells or induced subtelomeric DSBs relocalize to the NPC, though intact telomeres are normally tethered to the nuclear periphery by Mps3 (Therizols et al.2006; Bupp et al.2007; Khadaroo et al.2009; Schober et al.2009; Chung et al.2015). The lesions that have been found to relocate to the yeast nuclear pore or NE and the circumstances required are summarized in Tables 1 and 2, respectively.
Table 1.
Interactions at the NPC and functional consequences.
| Lesion type | NPC component associated with lesion | Cell cycle phase of association | Mediators (NPC interaction dependent on) | Function of relocalization | Reference |
|---|---|---|---|---|---|
| Persistent DSB (HO | Nup84 (ChIP) | G1/S/G2 | Slx5/8 | Increase survival | Nagai et al. (2008); |
| break, no donor for | Nup133 (ChIP) | Mec1/Tel1 | Promote gene conversion | Kalocsay, Hiller and | |
| repair) | Nic96 (ChIP) | Swr1 | Promote ectopic BIR | Jentsch (2009); Oza | |
| Nup49 (Imaginga, b) | Mms21 | Promote MMEJ | et al. (2009); Horigome | ||
| Siz2 | Suppress GCRs | et al. (2014, 2016) | |||
| Smc5/6e | |||||
| Subtelomeric DSB | Nup84 (ChIP) | N/D | Kinesin14 (Cik1, Kar3) | Increase survival | Therizols et al. (2006); |
| Cohibin (Lrs4, Csm1) | Promote end joiningf | Chung et al. (2015) | |||
| Swr1e | Promote Rad52-dependent BIR | ||||
| Eroded telomere | Nup49 (ChIP, | Senescing | Slx5/8 | Relocalize to pores from NE | Khadaroo et al. (2009); |
| (Imaginga) | cells | Siz1/Siz2 | Promote Rad52-dependent | Churikov et al. (2016) | |
| Rad9/Rad24e | type II recombination | ||||
| Collapsed fork | Nup49 (Imaginga, b) | S | - | Increase survival | Nagai et al. (2008) |
| by HU + MMSc | Promote fork restart | ||||
| Collapsed fork at | Nup49 (Imagingb) | S | Nup84 | Reduce repeat breakage and | Su et al. (2015) |
| CAG repeatsd | Nup84 (ChIP) | Slx5/8 | instability | ||
| Suppress Rad52-dependent HR |
Colocalization of fluorescently tagged pore protein with the lesion in either wild-type or nup133ΔN mutant cells (which clusters NPCs to one side of the nucleus; Doye, Wepf and Hurt 1994).
Preferential localization of the lesion at the periphery of the nucleus (zone 1) by zoning analysis.
Induced collapsed fork by treatment with 0.2 M HU and 0.03% MMS.
(CAG)70 or (CAG)130 repeat tracts.
Mutant causes partial delocalization.
Concluded to be NHEJ in Therizols et al. (2006), but a significant fraction (at least 40%) had what is now accepted as a MMEJ signature.
Table 2.
Interactions at the NE and functional consequences.
| Lesion type | NE component associated with lesion | Cell cycle phase of association | Mediators (NE interaction dependent on) | Function of tethering | Reference |
|---|---|---|---|---|---|
| Persistent DSB (HO break, no donor for repair) | Mps3 (ChIP, Imaginga) Heh2 (ChIP) | S/G2 | Rad51, Rad52, Rad9/Rad24, H2A.Zc, Swr1, INO80 (Arp8) SMC5/6 (Nse5), Mms21, Rtt107 | Delays HR repair Repress uSCR Repress HR with an ectopic donor Promote GCRs in NP mutants Recruit telomerase | Kalocsay, Hiller and Jentsch (2009); Oza et al. (2009); Horigome et al. (2014, 2016) |
| Cdc13 | |||||
| Slowly repaired DSB (30 kb resection required for SSA) | Mps3 (ChIP) | N/D | N/D | Unclear (repair is dependent on Rad52, partially on Nup84) | Oza et al. (2009); Chung et al. (2015) |
| Repairable DSB (HO break, ectopic donor on different chromosome) | Mps3 (ChIPb, Imaginga) | N/D | N/D | Suppress HR with an ectopic donor | Oza et al. (2009); Horigome et al. (2014) |
Preferential localization of the lesion at the periphery of the nucleus (zone 1) is lost in the mps3ΔN mutant.
Repairable DSBs do not show a zone 1 increase by imaging or bind to Mps3 by ChIP (Nagai et al.2008; Oza et al.2009).
H2A.Z is encoded by the HTZ1 gene; NE interaction is also lost in htz1-K126R, K133R non-sumoylatable mutants (Kalocsay, Hiller and Jentsch 2009) or the htz1ΔM6 mutant that doesn't bind SWR-C, but retains its Mps3 inner nuclear membrane localization function (Gardner et al.2011; Horigome et al.2014).
Besides DSBs, and eroded telomeres, collapsed replication forks relocalize to the nuclear periphery, specifically the nuclear pore (Nagai et al.2008; Su et al.2015). When replication forks stall, multiple outcomes are possible. A fork stalled by depletion of nucleotides (for example by, treatment with hydroxyurea (HU)) or encountering a barrier can retain an intact replisome for at least some period of time. In yeast, the replisome is still associated with forks stalled for 1 h in HU, and forks can restart replication if the HU is removed (Cobb et al.2005). These HU stalled forks do not relocate to the nuclear pore (Nagai et al.2008; Su et al.2015). Forks that encounter a nick (induced by the Flp-nick system) also remain in the interior of the nucleus (Dion et al.2012). On the other hand, prolonged incubation in HU or pretreatment with the DNA alkylating agent methylmethane sulfonate (MMS) before release into HU, conditions that induce repair foci, triggers relocation (Nagai et al.2008). Recently, we found that forks that encounter expanded CAG repeats of 70–130 units transiently move to the nuclear periphery in late S phase, interacting with Nup84 before disengaging by G2 phase (Su et al.2015). Chromosomes with longer repeats relocate more frequently. Repeats expanded to this range accumulate Rad52 foci and cause a checkpoint response that is mild but measurable in a significant proportion of cells, with a more severe response in a subset of cells (Gellon et al.2011; Sundararajan and Freudenreich 2011). The level and transient nature of the relocation to the NPC argues that the perinuclear attachment is part of a normal cellular response to forks stalled at the expanded CAG tract, which can form secondary structures that interfere with replication and repair (Su et al.2015; Usdin, House and Freudenreich 2015).
An interesting question is, what is the state of the fork that provokes relocation? A collapsed fork is generally agreed to be one that has lost replication competence, which in most cases probably also includes loss of a functional replisome (see Neelsen and Lopes 2015 for review). In the above cases, the stalled fork may be unable to restart effectively due to excessive damaged bases or hairpin structures. A collapsed fork may be converted to a broken fork, for example, by the action of structure-specific nucleases acting on the stalled fork. However, this step may not be the key factor in determining relocation since the broken fork caused by replication encountering a nick does not provoke the response, and pore association of the CAG tract actually prevents repeat fragility. Another factor that could play a role is fork reversal. If leading and lagging strand synthesis becomes uncoupled, the fork can regress, perhaps aided by annealing of the two nascent strands, to form a four-way junction or chicken foot structure visible by electron microscopy (see Neelsen and Lopes 2015 for review). In yeast, fork reversal has been shown to occur at uncoupled forks with extensive single-strand DNA (ssDNA) regions, for example, in cells that are checkpoint defective (Lopes et al.2001; Sogo, Lopes and Foiani 2002), or undergoing topological stress (Ray Chaudhuri et al.2012). In addition, there is experimental evidence for fork reversal occurring naturally in unperturbed cells at both CAG and GAA repeats, using direct visualization of replication intermediates by 2D gel electrophoresis and electron microscopy (Fouche et al.2006; Kerrest et al.2009; Follonier et al.2013). Although fork reversal can be a transient response to replication stress that facilitates lesion bypass, it could also lead to ‘stuck’ intermediates that are resistant to fork remodeling and restart (Fig. 2; collapsed fork).
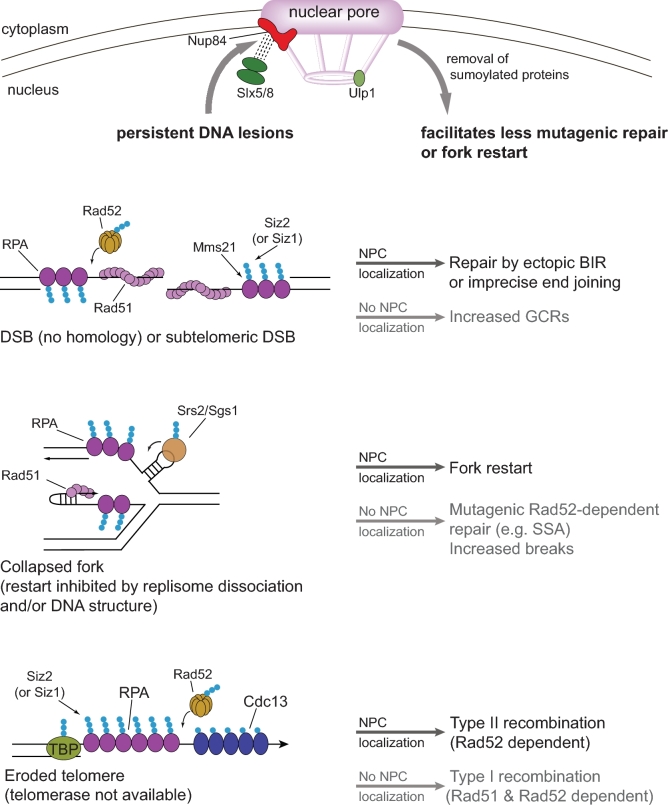
Figure 2.
Summary of lesions found to relocate to the nuclear pore, some sumoylated proteins expected to bind them and observed functional outcomes of relocation. At top is a model for the function of relocation of persistent DNA lesions to the nuclear pore. The dashed line between Slx5/Slx8 STUbL and the Y-shaped Nup84 complex indicates interaction between Nup84 and Slx8 shown by Nagai et al. (2008). The isopeptidase Ulp1 that can cleave SUMO from modified proteins interacts with the nuclear basket. Three types of persistent DNA lesions that have been studied are illustrated at left. Only a few key proteins are shown; other proteins that have been shown to interact with these lesions and/or play a role in mediating interaction with the nuclear pore or NE are listed in Tables 1 and 2. The small blue circles represent SUMO (either mono-SUMO or poly-SUMO chains). TBP stands for telomere binding proteins. On the right are listed the known outcomes of interaction with the nuclear pore for each type of lesion (black arrows), or alternative outcomes that occur when NPC interaction is defective (gray arrows).
MECHANISMS OF DNA DAMAGE RELOCATION
Once one of the persistent lesions discussed above forms, how does it trigger relocation to the nuclear pore? A common theme emerging is that sumoylation pathways play a key role in mediating the interaction (Table 1). SUMO (small ubiquitin-like modifier) modifies lysine residues of its target substrates. Covalent addition of SUMO (encoded by the SMT3 gene in yeast) is achieved by SUMO ligases: in yeast the only E2 ligase is Ubc9, and there are three E3 ligases that provide specificity, Siz1, Siz2 and Mms21, all part of the PIAS family also found in mammalian cells (Sarangi and Zhao 2015). SUMO can be removed by the SUMO-specific isopeptidases Ulp1 and Ulp2; Ulp1 is located at the nuclear pore (Li and Hochstrasser 1999; Zhao, Wu and Blobel 2004) (Fig. 2). Many DNA repair proteins are sumoylated, and several proteomic studies have identified sumoylated proteins present under normal or DNA damaging conditions (see for example Cremona et al.2012; Psakhye and Jentsch 2012; Albuquerque et al.2013; Thu et al.2016). Typically, levels of sumoylated proteins are very low as only a small percentage is modified. There is evidence that sumoylation is an ‘on-site’ modification that only occurs when the target protein is bound to DNA damage (Sarangi and Zhao 2015). Some notable DNA repair proteins known to be sumoylated in both yeast and human cells and present at breaks or collapsed forks are Rad52, RPA (Rfa1-3), Mre11, Sgs1, Srs2, Cdc13 and Smc5/6, but there are many others (Galanty et al.2009; Cremona et al.2012; Psakhye and Jentsch 2012; Sarangi and Zhao 2015; Thu et al.2016).
The Slx5/8 complex and its human homolog RNF4 are SUMO-targeted Ubiquitin Ligases (STUbLs) that contain multiple SUMO-interacting motifs (SIM domains). The Slx5/8 complex provides an intriguing link between on-site sumoylation at DNA lesions and relocation to the NPC, as it interacts with Nup84, and mediates relocation of each of the lesions shown to interact with the nuclear pore tested to date, including an expanded CAG tract, eroded telomere and persistent DSB (Table 1) (Nagai et al.2008; Su et al.2015; Churikov et al.2016; Horigome et al.2016). Both Ulp1 and the proteasome (which targets ubiquitylated substrates) are also located at the NPC, which could facilitate either desumoylation or degradation of sumoylated proteins that relocate there (Geli and Lisby 2015). The Slx5/8 homolog RNF4 mediates chromatin removal of MDC1, RPA, FANCI and FANCD2, which are important sensors of stalled forks and replication stress (Galanty et al.2012; Gibbs-Seymour et al.2015). Similarly, deletion or mutation of the Saccharomyces cerevisiae or Schizosaccharomyce pombe SLX5 or SLX8 genes render cells hypersensitive to HU (Mullen et al.2001; Zhang et al.2006; Prudden et al.2007).
One model for how Slx5/8-mediated chromosome relocation could work (Fig. 2) is that upon DNA damage, sumoylated repair proteins accumulate at the lesion site, which would bind Slx5/8 via the SIM domains in Slx5 (Xie et al.2007; Horigome et al.2016). Slx5/8 would then mediate interaction with the pore by its ability to bind Nup84 (Nagai et al.2008). Recent work from the Gasser lab showed that monosumoylation by Mms21, a component of Smc5/6, a cohesion-like complex, is sufficient for relocation of a DSB to Mps3, but that polysumoylation by Siz2 is needed for relocation to the NPC in G1 phase. Eroded telomeres were shown by Smt3 chromatin immunoprecipitation (ChIP) to accumulate SUMO-modified proteins coincident with cell senescence (when telomeres are short) as well as with Slx8 binding (Churikov et al.2016). In addition, telomere relocation to the NPC was impaired by deletion of Slx8, Siz1 or Siz2, though slx8Δ had the greatest effect. Lastly, RPA is polysumoylated during senescence, and physically interacts with Slx5/8 (Chung and Zhao 2015; Churikov et al.2016), making it a prime target for mediating the interactions necessary for relocation to the NPC (Fig. 2). Whether this model will apply to collapsed forks relocating in S phase is less clear. Slx5/8 was important for CAG tract relocation, although the defect was not as great as in a nup84Δ (Su et al.2015). However, Nup84 has additional functions beyond interaction with Slx5/8, for example, it is required for stabilization of Ulp1 and its association with the NPC (Palancade et al.2007), and this defect could contribute to the delocalization of the CAG tract. Also, Slx5/8 is only partially required for DSB–pore interaction in S phase, even though it is fully required in G1 (Horigome et al.2016).
There is also evidence for involvement of other mechanisms in relocation to the NPC in addition to SUMO interactions. Deletion of several other genes was shown to disrupt NPC localization of DSBs or eroded telomeres, including Swr1, Mec1/Tel1 and Rad9/Rad24 (Table 1). One thing all these proteins have in common is that they have been shown to be important in DSB mobility that occurs after break induction (Dion et al.2012; Mine-Hattab and Rothstein 2012; Horigome et al.2014). Interestingly, the checkpoint proteins Mec1 and Rad53 also play a role in stabilizing stalled replication forks that encounter active transcription, which is hypothesized to occur by release of transcribing genes from basket nucleoporins to reduce DNA topological tension (Bermejo et al.2011). It is not known whether these functions are related to their role in NPC localization. Another player is the Smc5/6 complex. The Nse5 subunit, which facilitates Smc5/6 recruitment to sites of replication stress (Cook, Hochstrasser and Kerscher 2009; Bustard et al.2012), can interact directly with Slx5, and ablation of this interaction reduced interaction with Mps3, though it had a more minor role in pore association (Horigome et al.2016). The Slx5-Smc5/6 interaction is conserved in S. pombe (Prudden et al.2007), and the Smc5/6 complex, along with another STUbL interacting complex RENi (Rad60, Esc2, Nip45) is necessary for relocation of heterchromatic DSBs in flies (Chiolo et al.2011; Ryu et al.2015). Even more proteins have been implicated in DSB localization to Mps3, including the telomere binding protein Cdc13 (Table 2). Recently, it was found that a subtelomeric DSB moves from the NE location normally occupied by telomeres to the NPC, and this movement was dependent on cohibin, a telomere tethering complex, kinesin14, a motor protein complex and α-tubulin (Chung et al.2015). The authors propose that an active microtubule-motor process moves the damaged telomere between sites. Thus, there may be multiple overlapping systems that recruit damaged DNA to the nuclear periphery, and determine the sites of interaction.
FUNCTIONAL CONSEQUENCES OF DAMAGE RELOCATION
An important question is, what is the purpose of relocation to the nuclear pore or NE? This question has been addressed by monitoring DNA repair or telomere addition in mutants defective in relocation. Another strategy that has been employed is to artificially tether DNA to the pore and determine whether repair outcome changes using a genetic reporter assay. Functions of perinuclear tethering at each type of lesion that have been surmised from these approaches are summarized in Tables 1 and 2. Though there are still many questions, some themes are emerging. One fairly consistent result is that tethering at the NE by Mps3 generally suppresses HR, indicating that it is likely a repair repressive environment (Table 2). In contrast, the NPC appears to be a more permissive environment, promoting several types of repair (Table 1). However, there appear to be subtleties about which types of repair are promoted or suppressed by NPC localization, which may vary somewhat by lesion type or cell cycle phase. The most consistent theme is that a persistent lesion, which has failed to repair by a preferred or conservative mechanism, may relocate to the NPC to allow an alternative mechanism of repair to occur. This generally promotes survival and promotes repair, but at the expense of genome stability (Fig. 2).
For example, for a DSB, the preferred pathway of repair would usually be Rad51-dependent HR with a sister chromatid, or non-homologous end joining (NHEJ), which leads to minimal mutations (Symington and Gautier 2011). However, alternate pathways available are repair from an ectopic site, which can result in loss of heterozygosity, or microhomology-mediated end joining (MMEJ), which often results in large deletions or insertions (Sfeir and Symington 2015; Rodgers and McVey 2016). For a one-ended break, such as would occur at an eroded telomere or collapsed fork, break-induced replication using another chromosome as the template (ectopic BIR) would be an alternative rescue mechanism if telomere addition and fork restart on the same chromosome were not functioning.
The data available to date suggest that NPC localization is facilitating just such ‘alternate’ repair choices that may be mutagenic but are a preferred alternative to death or gross chromosomal rearrangements (GCRs) (Fig. 2). For example, for a persistent DSB, it was found that ectopic BIR and MMEJ were reduced in slx8 or nup84 mutants, and cell death and GCRs increased (Nagai et al.2008; Horigome et al.2016). For a subtelomeric DSB, survivors usually used BIR to repair, which was reduced in mutants with defective NPC localization, and artificial tethering to the pore hyperactivated BIR (Chung et al.2015). Pore-dependent BIR events assayed with a variety of reporters were all highly dependent on Rad52 and Pol32, two proteins previously established to be necessary for ectopic BIR (Anand, Lovett and Haber 2013; Chung et al.2015). Eroded telomeres can be rescued by either type I or type II recombination, with type II being more prominent in survivors; however, NPC mutants exhibit severely reduced numbers of type II recombinants (Churikov et al.2016). Indeed, tethering of an eroded telomere to Nup60 resulted in a significant increase in type II recombination events, providing direct evidence for a role for the NPC location in stimulating this event (Churikov et al.2016). Recently, Nup60 was found to be both monoubiquitylated and sumoylated; ubiquitylated Nup60 interacts with the Nup84 complex to tether it to the NPC and contributes to the cellular response to DNA damage (Nino et al.2016).
Consistently, a ubiquitin-deficient mutant of Nup60 favors the maintenance of type I survivors (Nino et al.2016).
The NPC-dependent events that occur at a collapsed fork are less clear. In common with other lesions, relocation has a positive effect on survival, as it prevents breaks at the expanded CAG repeat (since repeat-associated chromosome end loss events are increased in the absence of Slx5/8 or Nup84) (Su et al.2015). In addition, CAG expansions and contractions are increased in slx5/8 or nup84 mutants, and instability was caused by a process with greater dependence on Rad52 than Rad51 (Fig. 2). Thus, the data suggest that NPC association inhibited a mutagenic Rad52-dependent pathway or pathways that are prone to causing repeat instability, such as mutagenic SSA or another recombination pathway that can occur by Rad52-dependent annealing. Alternatively, it may be that for a collapsed fork, the preferred alternative pathway facilitated by NPC association is HR-dependent fork restart, but that some type of ‘release’ on recombination is required for restart to occur efficiently at a ‘stuck’ fork.
Rad52 sumoylation is induced upon DNA damage by MMS, and sumoylated Rad52 is ubiquitylated by Slx5/8 (Sacher et al.2006; Burgess et al.2007; Xie et al.2007). Interestingly, in the case of collapsed forks, degradation of a Rad52-Smt3 fusion protein (mimicking monosumoylation at the C-terminus) occurred coincident with NPC association, in a manner dependent on Slx8 (Su et al.2015). Rad52 sumoylation regulates repair efficiency of several different repair pathways. For example, in cells containing a mutant Rad52 protein lacking sumoylation sites, a shift from SSA to gene conversion, an increase in direct and inverted repeat recombination and a reduction in BIR and other types of interchromosomal events were observed (Altmannova et al.2010; Silva et al.2016). In addition, the Rad52 paralog Rad59 has two sites of sumoylation, and Rad52 and Rad59 sumoylation appear to act in synergy, with double mutants showing more pronounced phenotypes compared to the singles. The authors propose that the non-sumoylated forms of these proteins promote Rad51-dependent intrachromosomal types of recombination, while events that require more robust annealing (such as BIR or interchromosomal recombination) are promoted by the sumoylated forms (Silva et al.2016). Thus, regulation of Rad52 sumoylation by NPC relocation is one mechanism that could influence repair outcomes.
CONCLUSIONS
These studies in yeast have elucidated an important role for movement of chromosomes between different nuclear compartments to facilitate DNA repair and regulate pathway choice. Several available tools in yeast have allowed this field to advance, including genetic knockouts, the ability to mark chromosomes and proteins fluorescently to follow their nuclear location and mobility, inducible systems for particular types of DNA damage, ease of cell cycle analysis and tools to tether particular chromosome regions to protein complexes. In addition, a wealth of assays for different repair pathways and outcomes (unequal sister chromatid recombination, BIR, NHEJ, MMEJ, SSA, GCRs, telomere rescue pathways, etc.) have allowed researchers to correlate localization to different nuclear compartments with repair outcomes. Importantly, the findings from yeast appear to be highly relevant to understanding similar events in other types of eukaryotic cells, though differences are also evident. For example, results from the Chiolo lab found that in Drosophila cells, heterochromatic DSBs move to the nuclear periphery before undergoing HR repair, a protective mechanism that prevents ectopic recombination (Chiolo et al.2011; Ryu et al.2015). This relocalization also depends on nuclear pore and inner nuclear membrane proteins that anchor repair sites to the nuclear periphery (Ryu et al.2015). Furthermore, it appears that this DSB targeting also relies on SUMO and SUMO E3 ligases (Ryu et al.2015). There is also compartmentalization of DNA repair in mammalian cells (see Lemaitre and Soutoglou 2015 for review). Similar to the situation in yeast, the nuclear lamina appears to be a repressive environment for HR (with NHEJ or MMEJ preferred), whereas the NPC is permissive for HR (Lemaitre et al.2014). However, in this case, movement between the lamina and the NPC was not detected. On the other hand, Lamin A/C depletion causes sensitivity to agents that stall forks (HU, interstrand cross-linking agents) and defective fork restart (Singh et al.2013), so it appears that regulation of both DSB repair and fork restart may be occurring at perinuclear domains in mammalian cells, as in yeast. The association between nuclear periphery components and fork restart in human cells, combined with the parallels between yeast Slx5/8 and human RNF4 (which interacts with sensors of stalled forks), suggests that relocation of collapsed forks may also be important to prevent genome instability in human cells. Expanded triplet repeats are a physiologically relevant site of fork stalling that have been shown to perturb replication in human cells (Voineagu et al.2009; Cleary et al.2010; Liu and Leffak 2012), and thus could be dependent on such a relocation process to prevent further expansions or chromosome breaks at the repeat. Also, mutations in Lamin A cause a degenerative disease marked by premature aging, Hutchinson-Gilford progeria syndrome, and patient cells exhibit accumulation of DNA damage, defects in DNA repair, genomic instability and telomere shortening (Gonzalo, Kreienkamp and Askjaer 2016). Mutations in human nucleoporin genes have been reported in a variety of inherited diseases and cancers, but links to defects in DNA repair have not yet been made (Nofrini, Di Giacomo and Mecucci 2016). Overall, these examples indicate that the connection between DNA repair, fork restart and perinuclear location is likely to be conserved across species, even if some details may prove to be different. The data gained from studies in yeast over the last decade, using various types of induced DNA damage along with informative genetic assays and imaging approaches, provide a framework for understanding the interplay between nuclear repositioning and regulation of DNA repair.
FUNDING
The researchers were funded by Tufts University and NIH grant P01GM105473 to CHF.
Conflict of interest. None declared.
REFERENCES
- Albuquerque CP, Wang G, Lee NS et al. Distinct SUMO ligases cooperate with Esc2 and Slx5 to suppress duplication-mediated genome rearrangements. PLoS Genet 2013;9:e1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmannova V, Eckert-Boulet N, Arneric M et al. Rad52 SUMOylation affects the efficiency of the DNA repair. Nucleic Acids Res 2010;38:4708–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand RP, Lovett ST, Haber JE. Break-induced DNA replication. Cold Spring Harb Perspect Biol 2013;5:a010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniacci LM, Kenna MA, Skibbens RV. The nuclear envelope and spindle pole body-associated Mps3 protein bind telomere regulators and function in telomere clustering. Cell Cycle 2007;6:75–9 [DOI] [PubMed] [Google Scholar]
- Bennett CB, Lewis LK, Karthikeyan G et al. Genes required for ionizing radiation resistance in yeast. Nat Genet 2001;29:426–34 [DOI] [PubMed] [Google Scholar]
- Bermejo R, Capra T, Jossen R et al. The replication checkpoint protects fork stability by releasing transcribed genes from nuclear pores. Cell 2011;146:233–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bupp JM, Martin AE, Stensrud ES et al. Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J Cell Biol 2007;179:845–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RC, Rahman S, Lisby M et al. The Slx5-Slx8 complex affects sumoylation of DNA repair proteins and negatively regulates recombination. Mol Cell Biol 2007;27:6153–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustard DE, Menolfi D, Jeppsson K et al. During replication stress, non-SMC element 5 (NSE5) is required for Smc5/6 protein complex functionality at stalled forks. J Biol Chem 2012;287:11374–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolo I, Minoda A, Colmenares SU et al. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 2011;144:732–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DK, Chan JN, Strecker J et al. Perinuclear tethers license telomeric DSBs for a broad kinesin- and NPC-dependent DNA repair process. Nat Commun 2015;6:7742. [DOI] [PubMed] [Google Scholar]
- Chung I, Zhao X. DNA break-induced sumoylation is enabled by collaboration between a SUMO ligase and the ssDNA-binding complex RPA. Gene Dev 2015;29:1593–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churikov D, Charifi F, Eckert-Boulet N et al. SUMO-dependent relocalization of eroded telomeres to nuclear pore complexes controls telomere recombination. Cell Rep 2016;15:1242–53 [DOI] [PubMed] [Google Scholar]
- Cleary JD, Tome S, Lopez Castel A et al. Tissue- and age-specific DNA replication patterns at the CTG/CAG-expanded human myotonic dystrophy type 1 locus. Nat Struct Mol Biol 2010;17:1079–87 [DOI] [PubMed] [Google Scholar]
- Cobb JA, Schleker T, Rojas V et al. Replisome instability, fork collapse, and gross chromosomal rearrangements arise synergistically from Mec1 kinase and RecQ helicase mutations. Gene Dev 2005;19:3055–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CE, Hochstrasser M, Kerscher O. The SUMO-targeted ubiquitin ligase subunit Slx5 resides in nuclear foci and at sites of DNA breaks. Cell Cycle 2009;8:1080–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona CA, Sarangi P, Yang Y et al. Extensive DNA damage-induced sumoylation contributes to replication and repair and acts in addition to the mec1 checkpoint. Mol Cell 2012;45:422–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion V, Gasser SM. Chromatin movement in the maintenance of genome stability. Cell 2013;152:1355–64 [DOI] [PubMed] [Google Scholar]
- Dion V, Kalck V, Horigome C et al. Increased mobility of double-strand breaks requires Mec1, Rad9 and the homologous recombination machinery. Nat Cell Biol 2012;14:502–9 [DOI] [PubMed] [Google Scholar]
- Doye V, Wepf R, Hurt EC. A novel nuclear pore protein Nup133p with distinct roles in poly(A)+ RNA transport and nuclear pore distribution. EMBO J 1994;13:6062–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follonier C, Oehler J, Herrador R et al. Friedreich's ataxia-associated GAA repeats induce replication-fork reversal and unusual molecular junctions. Nat Struct Mol Biol 2013;20:486–94 [DOI] [PubMed] [Google Scholar]
- Fouche N, Ozgur S, Roy D et al. Replication fork regression in repetitive DNAs. Nucleic Acids Res 2006;34:6044–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanty Y, Belotserkovskaya R, Coates J et al. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 2009;462:935–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanty Y, Belotserkovskaya R, Coates J et al. RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Gene Dev 2012;26:1179–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JM, Smoyer CJ, Stensrud ES et al. Targeting of the SUN protein Mps3 to the inner nuclear membrane by the histone variant H2A.Z. J Cell Biol 2011;193:489–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli V, Lisby M. Recombinational DNA repair is regulated by compartmentalization of DNA lesions at the nuclear pore complex. Bioessays 2015;37:1287–92 [DOI] [PubMed] [Google Scholar]
- Gellon L, Razidlo DF, Gleeson O et al. New functions of Ctf18-RFC in preserving genome stability outside its role in sister chromatid cohesion. PLoS Genet 2011;7:e1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs-Seymour I, Oka Y, Rajendra E et al. Ubiquitin-SUMO circuitry controls activated fanconi anemia ID complex dosage in response to DNA damage. Mol Cell 2015;57:150–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo S, Kreienkamp R, Askjaer P. Hutchinson-Gilford Progeria syndrome: a premature aging disease caused by LMNA gene mutations. Ageing Res Rev 2016pii: S1568–163730134–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y, Foisner R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem 2015;84:131–64 [DOI] [PubMed] [Google Scholar]
- Horigome C, Bustard DE, Marcomini I et al. PolySUMOylation by Siz2 and Mms21 triggers relocation of DNA breaks to nuclear pores through the Slx5/Slx8 STUbL. Gene Dev 2016;30:931–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horigome C, Oma Y, Konishi T et al. SWR1 and INO80 chromatin remodelers contribute to DNA double-strand break perinuclear anchorage site choice. Mol Cell 2014;55:626–39 [DOI] [PubMed] [Google Scholar]
- Jakob B, Splinter J, Conrad S et al. DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin. Nucleic Acids Res 2011;39:6489–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen SL, Giddings TH Jr, Winey M. Mps3p is a novel component of the yeast spindle pole body that interacts with the yeast centrin homologue Cdc31p. J Cell Biol 2002;159:945–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalocsay M, Hiller NJ, Jentsch S. Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Mol Cell 2009;33:335–43 [DOI] [PubMed] [Google Scholar]
- Kelley K, Knockenhauer KE, Kabachinski G et al. Atomic structure of the Y complex of the nuclear pore. Nat Struct Mol Biol 2015;22:425–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrest A, Anand RP, Sundararajan R et al. SRS2 and SGS1 prevent chromosomal breaks and stabilize triplet repeats by restraining recombination. Nat Struct Mol Biol 2009;16:159–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadaroo B, Teixeira MT, Luciano P et al. The DNA damage response at eroded telomeres and tethering to the nuclear pore complex. Nat Cell Biol 2009;11:980–7 [DOI] [PubMed] [Google Scholar]
- Lemaitre C, Grabarz A, Tsouroula K et al. Nuclear position dictates DNA repair pathway choice. Gene Dev 2014;28:2450–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre C, Soutoglou E. DSB (Im)mobility and DNA repair compartmentalization in mammalian cells. J Mol Biol 2015;427:652–8 [DOI] [PubMed] [Google Scholar]
- Li SJ, Hochstrasser M. A new protease required for cell-cycle progression in yeast. Nature 1999;398:246–51 [DOI] [PubMed] [Google Scholar]
- Lisby M, Rothstein R, Mortensen UH. Rad52 forms DNA repair and recombination centers during S phase. P Natl Acad Sci USA 2001;98:8276–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M, Teixeira T, Gilson E et al. The fate of irreparable DNA double-strand breaks and eroded telomeres at the nuclear periphery. Nucleus 2010;1:158–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Leffak M. Instability of (CTG)n*(CAG)n trinucleotide repeats and DNA synthesis. Cell Biosci 2012;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeillet S, Palancade B, Cartron M et al. Genetic network interactions among replication, repair and nuclear pore deficiencies in yeast. DNA Repair 2005;4:459–68 [DOI] [PubMed] [Google Scholar]
- Lopes M, Cotta-Ramusino C, Pellicioli A et al. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 2001;412:557–61 [DOI] [PubMed] [Google Scholar]
- Lusk CP, Blobel G, King MC. Highway to the inner nuclear membrane: rules for the road. Nat Rev Mol Cell Bio 2007;8:414–20 [DOI] [PubMed] [Google Scholar]
- Mekhail K, Moazed D. The nuclear envelope in genome organization, expression and stability. Nat Rev Mol Cell Bio 2010;11:317–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine-Hattab J, Rothstein R. Increased chromosome mobility facilitates homology search during recombination. Nat Cell Biol 2012;14:510–7 [DOI] [PubMed] [Google Scholar]
- Mullen JR, Kaliraman V, Ibrahim SS et al. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics 2001;157:103–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S, Davoodi N, Gasser SM. Nuclear organization in genome stability: SUMO connections. Cell Res 2011;21:474–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S, Dubrana K, Tsai-Pflugfelder M et al. Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science 2008;322:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S, Heun P, Gasser SM. Roles for nuclear organization in the maintenance of genome stability. Epigenomics 2010;2:289–305 [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet 2009;43:525–58 [DOI] [PubMed] [Google Scholar]
- Neelsen KJ, Lopes M. Replication fork reversal in eukaryotes: from dead end to dynamic response. Nat Rev Mol Cell Bio 2015;16:207–20 [DOI] [PubMed] [Google Scholar]
- Nino CA, Guet D, Gay A et al. Posttranslational marks control architectural and functional plasticity of the nuclear pore complex basket. J Cell Biol 2016;212:167–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Terazawa Y, Nakayama T et al. Nep98p is a component of the yeast spindle pole body and essential for nuclear division and fusion. J Biol Chem 2003;278:9938–43 [DOI] [PubMed] [Google Scholar]
- Nofrini V, Di Giacomo D, Mecucci C. Nucleoporin genes in human diseases. Eur J Hum Genet 2016;24:1388–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza P, Jaspersen SL, Miele A et al. Mechanisms that regulate localization of a DNA double-strand break to the nuclear periphery. Gene Dev 2009;23:912–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palancade B, Liu X, Garcia-Rubio M et al. Nucleoporins prevent DNA damage accumulation by modulating Ulp1-dependent sumoylation processes. Mol Biol Cell 2007;18:2912–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudden J, Pebernard S, Raffa G et al. SUMO-targeted ubiquitin ligases in genome stability. EMBO J 2007;26:4089–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psakhye I, Jentsch S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 2012;151:807–20 [DOI] [PubMed] [Google Scholar]
- Ray Chaudhuri A, Hashimoto Y, Herrador R et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat Struct Mol Biol 2012;19:417–23 [DOI] [PubMed] [Google Scholar]
- Rodgers K, McVey M. Error-prone repair of DNA double-strand breaks. J Cell Physiol 2016;231:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu T, Spatola B, Delabaere L et al. Heterochromatic breaks move to the nuclear periphery to continue recombinational repair. Nat Cell Biol 2015;17:1401–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M, Pfander B, Hoege C et al. Control of Rad52 recombination activity by double-strand break-induced SUMO modification. Nat Cell Biol 2006;8:1284–90 [DOI] [PubMed] [Google Scholar]
- Sarangi P, Zhao X. SUMO-mediated regulation of DNA damage repair and responses. Trends Biochem Sci 2015;40:233–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober H, Ferreira H, Kalck V et al. Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Gene Dev 2009;23:928–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TU. The structure inventory of the nuclear pore complex. J Mol Biol 2016;428:1986–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Symington LS. Microhomology-mediated end joining: a back-up survival mechanism or dedicated pathway?. Trends Biochem Sci 2015;40:701–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S, Altmannova V, Eckert-Boulet N et al. SUMOylation of Rad52-Rad59 synergistically change the outcome of mitotic recombination. DNA Repair 2016;42:11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Hunt CR, Pandita RK et al. Lamin A/C depletion enhances DNA damage-induced stalled replication fork arrest. Mol Cell Biol 2013;33:1210–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo JM, Lopes M, Foiani M. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 2002;297:599–602 [DOI] [PubMed] [Google Scholar]
- Sood V, Brickner JH. Nuclear pore interactions with the genome. Curr Opin Genet Dev 2014;25:43–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su XA, Dion V, Gasser SM et al. Regulation of recombination at yeast nuclear pores controls repair and triplet repeat stability. Genes and Development 2015;29:1006–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan R, Freudenreich CH. Expanded CAG/CTG repeat DNA induces a checkpoint response that impacts cell proliferation in Saccharomyces cerevisiae. PLoS Genet 2011;7:e1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington LS, Gautier J. Double-strand break end resection and repair pathway choice. Annu Rev Genet 2011;45:247–71 [DOI] [PubMed] [Google Scholar]
- Symington LS, Rothstein R, Lisby M. Mechanisms and regulation of mitotic recombination in Saccharomyces cerevisiae. Genetics 2014;198:795–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therizols P, Fairhead C, Cabal GG et al. Telomere tethering at the nuclear periphery is essential for efficient DNA double strand break repair in subtelomeric region. J Cell Biol 2006;172:189–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu YM, Van Riper SK, Higgins L et al. Slx5/Slx8 promotes replication stress tolerance by facilitating mitotic progression. Cell Rep 2016;15:1254–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rosell J, Sunjevaric I, De Piccoli G et al. The Smc5-Smc6 complex and SUMO modification of Rad52 regulates recombinational repair at the ribosomal gene locus. Nat Cell Biol 2007;9:923–31 [DOI] [PubMed] [Google Scholar]
- Tsouroula K, Furst A, Rogier M et al. Temporal and spatial uncoupling of DNA double strand break repair pathways within mammalian heterochromatin. Mol Cell 2016;63:293–305 [DOI] [PubMed] [Google Scholar]
- Usdin K, House NC, Freudenreich CH. Repeat instability during DNA repair: insights from model systems. Crit Rev Biochem Mol 2015;50:142–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineagu I, Surka CF, Shishkin AA et al. Replisome stalling and stabilization at CGG repeats, which are responsible for chromosomal fragility. Nat Struct Mol Biol 2009;16:226–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Kerscher O, Kroetz MB et al. The yeast Hex3.Slx8 heterodimer is a ubiquitin ligase stimulated by substrate sumoylation. J Biol Chem 2007;282:34176–84 [DOI] [PubMed] [Google Scholar]
- Zhang C, Roberts TM, Yang J et al. Suppression of genomic instability by SLX5 and SLX8 in Saccharomyces cerevisiae. DNA Repair 2006;5:336–46 [DOI] [PubMed] [Google Scholar]
- Zhao X, Wu CY, Blobel G. Mlp-dependent anchorage and stabilization of a desumoylating enzyme is required to prevent clonal lethality. J Cell Biol 2004;167:605–11 [DOI] [PMC free article] [PubMed] [Google Scholar]