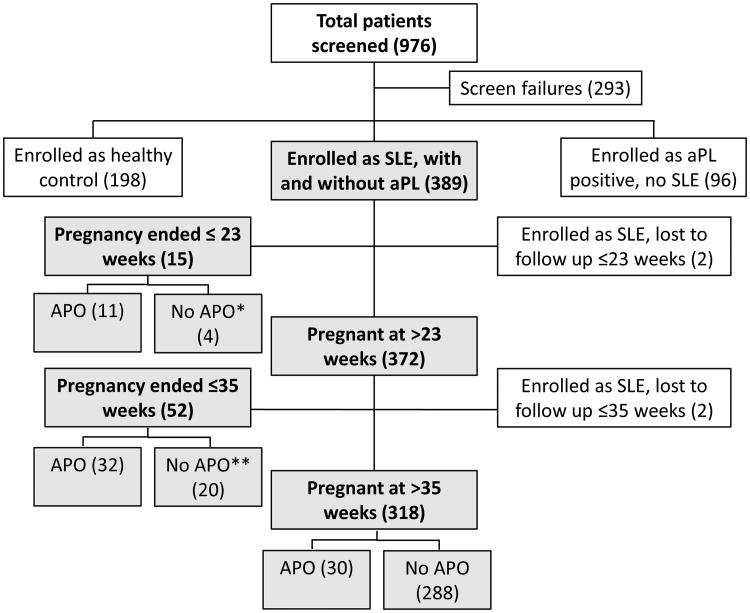
Appendix Figure 1. Patient enrollment and classification for the PROMISSE Study.
Pregnant women at <12 weeks' gestation with antiphospholipid antibody (aPL) positivity and systemic lupus erythematosus (SLE) and healthy controls were screened. Exclusion criteria included: multifetal pregnancy, prednisone >20 mg/day, blood pressure ≥140/90 mm Hgurine protein (mg)/creatinine (gram) ratio ≥1,000 mg protein/gm creatinine on 24-hour urine or spot urine collection, serum creatinine >1.2 mg/dl, screened too late in pregnancy, and previously enrolled in PROMISSE. Healthy pregnant women were enrolled if they had ≥ one successful pregnancy, no history of fetal death, and no more than 1 miscarriage <10 weeks' gestation and no antiphospholipid antibodies. The current study included all PROMISSE patients who met American College of Rheumatology criteria for systemic lupus erythematosus (SLE). Number of subjects is shown in parenthesis.
*Four patients whose pregnancy ended at <23 weeks did not meet study criteria for primary study outcome: elective termination (1), incompetent cervix (2), PPROM (1).See Table 1.
**Twenty patients whose pregnancy ended at <35 weeks did not meet study criteria for primary study outcome: indicated delivery for poor obstetric history (1), for SLE flare (1), for bleeding placental abruption (1); termination for complete heart block (1); fetal death due to trisomy 18 (1); PPROM and/or premature labor (15).See Table 1.
APO = adverse pregnancy outcome; aPL = antiphospholipid antibodies; PPROM = premature preterm rupture of membranes