Abstract
Animal models are used to study many human diseases, one of which is tobacco addiction. Most preclinical models use nicotine alone, although there are 7,000 constituents present in tobacco smoke. The clinical literature suggests that cigarettes have a strong addictive potential, which is not paralleled in preclinical studies using nicotine alone. In order to address the gap between clinical and preclinical literature on tobacco dependence, cigarette smoke extracts containing tobacco constituents have been developed. This unit describes a procedure for producing an aqueous cigarette smoke extract (CSE) which animals readily self-administer. In addition, we describe how to make the apparatus for producing CSE and how to analyze the solution for nicotine content.
Keywords: nicotine, self-administration, tobacco constituents, smoking
INTRODUCTION
Most preclinical models of nicotine addiction utilize nicotine alone because it is the main psychoactive constituent in tobacco (Harvey et al., 2004; Benowitz, 1988). However, when these models are used to investigate nicotine dependence, they have shown nicotine to be a weak reinforcer, which does not parallel the strong addictive potential of cigarettes (Mello and Newman, 2011; Manzardo et al., 2002). Cigarette smoke contains more than 7,000 constituents and their contribution to tobacco dependence has not been thoroughly studied (U.S. Surgeon General, 2010). Non-nicotine constituents in cigarette smoke have been shown to have reinforcing properties, as well as to additively enhance the reinforcing properties of nicotine (Arnold et al., 2014; Costello et al., 2014, Brennan et al., 2013; Belluzzi et al., 2005). Preclinical models used to access the abuse potential of cigarette smoke constituents include inhalation of cigarette smoke, and use of smokeless tobacco extracts, tobacco particulate matter extracts and cigarette smoke extract (CSE) (Costello et al., 2014; Brennan et al., 2013; Harris et al., 2010; Small et al., 2010). CSE is the only solution composed of all the aqueous constituents of mainstream (directly inhaled) cigarette smoke. Rodents reliably self-administer CSE, which allows for the assessment of abuse potential using preclinical models of acquisition, reward and reinforcement, as well as craving. CSE may also be used for both ex vivo and in vitro pharmacological tests. For a review on CSE pharmacology and its comparison to nicotine alone, see Costello et al. (2014).
Smoking machines have been manufactured to make reproducible samples of cigarette smoke extracts. However, these machines are costly, and require extensive maintenance. Here we describe a method of manufacturing CSE (Basic Protocol 1) with an inexpensive and easily assembled apparatus (Support Protocol 1). In addition, to accurately compare the abuse potential or pharmacological profile of CSE and nicotine alone, we describe a method to analyze the nicotine content in CSE (Support Protocol 2).
BASIC PROTOCOL 1: Preparing cigarette smoke extract
Introductory paragraph
The production of CSE samples is standardized for reproducible collection of nicotine and the aqueous constituents of cigarette smoke. With the use of an experimenter-operated syringe, puffs of defined volume (35 ml) are drawn from a lit cigarette (Camel unfiltered). Human smoking is modeled with short puffs (2 seconds) with long delays between puffs (30 seconds). The mainstream smoke, smoke that is drawn through the end of the cigarette during puffing, is bubbled through a saline solution. Eight cigarettes are “smoked” until a 23mm cigarette bud remains, producing a brown acidic solution that is adjusted to a physiological pH between 7.2 and 7.4 (see Video for full demonstration of preparing cigarette smoke extract).
Materials
Fume hood
CSE apparatus
Ring stand with 2 three-prong clamps
Camel unfiltered cigarettes (8)
Saline
50ml polypropylene conical tubes (2)
1000μl pipette tip
50ml glass syringe with glass plunger
1000ml glass beaker
Aluminum foil
Timer
Student Adson Forceps (12cm, serrated tip)
Black Fine Tip Permanent Marker
Petroleum Jelly
0.1 M, 1 M, and 10M NaOH
0.1 M, 1 M HCl
pH test strips
Protocol steps
-
Mark 8 cigarettes 23mm from the end with a permanent marker.
We use Camel unfiltered cigarettes and aim to produce a solution containing 150 μg/ml of nicotine. All cigarettes vary in nicotine content so depending on the brand of cigarettes and the target concentration, the number of cigarettes may vary.
-
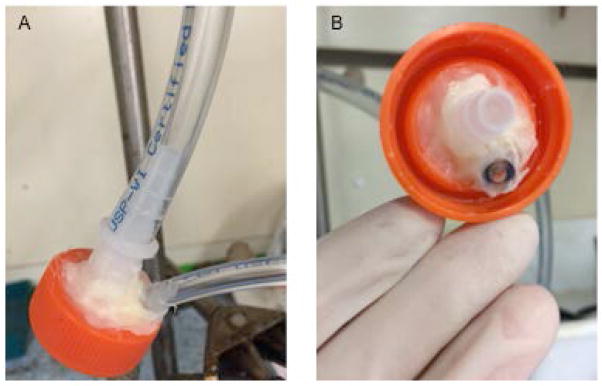
Attach a clean 1000μl pipette tip firmly to the inside of the apparatus which is located in a fume hood as shown in Figure 1 B.
This pipette tip will become clogged with tar and will need to be replaced after each batch of CSE.
All procedures should take place in a fume hood so that the experimenter and laboratory environment are not exposed to the cigarette smoke.
Fill a 50ml polypropylene conical tube with 35ml of saline, attach it to the apparatus as illustrated in Figure 1B.
-
Grease the sides of the 50ml glass plunger with petroleum jelly to ease the draws.
A single layer of petroleum jelly should be applied to the sides of the plunger that will be inserted into the syringe.
Make an ashtray with foil and place at the bottom of the cigarette location to catch falling ashes.
-
Attach the glass syringe to the small apparatus tubing as in Figure 1C, and insert the cigarette into the large tubing as in Figure 1A by carefully twisting and pushing, making sure the cigarette does not break and that there is a tight seal to prevent smoke leakage.
See Support Protocol 1 for dimensions of the small and large tubes.
-
Light the cigarette, start the timer and draw on the syringe up to 35ml. The puff should be slow and last 2–4 seconds.
With every draw, it is important to look around the apparatus to catch any smoke leaks. Any leaks will result in lower nicotine content than expected.
Detach the small tubing from the syringe and “exhale” the smoke in the syringe onto a paper towel.
Repeat 35ml puffs every 30 seconds.
Repeat until the marking on the cigarette is reached. Record the number of puffs; it should be 8 – 10.
-
Carefully remove the cigarette butt with the forceps.
If the cigarette butt does not come out in one piece, use the forceps to remove the remaining tobacco. The forceps should be small enough to fit around the cigarette bud to facilitate removal of all tobacco. If it is not, you must replace the large tubing.
Insert a new cigarette and repeat until all cigarettes are smoked.
-
When done, remove the conical tube from the apparatus and pour the extract into the clean, foil-wrapped 50ml polypropylene conical tube.
Tubing is wrapped in foil because nicotine is light sensitive. However, the tube in which CSE is made should not be wrapped in foil, as you will need to confirm that the smoke is bubbling through the saline.
-
Adjust the pH to between 7.2–7.4. It usually takes 4–5 drops of 1M NaOH. Test the pH with test strips.
Adjusting the pH of the solution to a neutral range is important for nicotine concentration analysis of CSE using an acid-base extraction (see Support Protocol 2) and for administration into an animal or biological tissue.
pH paper is preferred over a pH meter as the tar contents of CSE will damage the meter.
-
Store the CSE in the fridge for up to 24 hours.
Stability of the non-nicotine constituents is unknown so it is not recommended to use for longer than 24 hours after production.
-
Clean up: contain the ashtray in the dirty conical tube to keep the smell of cigarettes to a minimum. Place 1000ml beaker under apparatus in the fume hood and run hot water through the tubing using the 50ml glass syringe. Finish by cleaning the glass syringe with soap and water and allow it to dry overnight.
Make sure the apparatus is dry before making CSE again. Leftover water in the tubing may cause a loss of constituents and a decrease in nicotine content.
Figure 1.
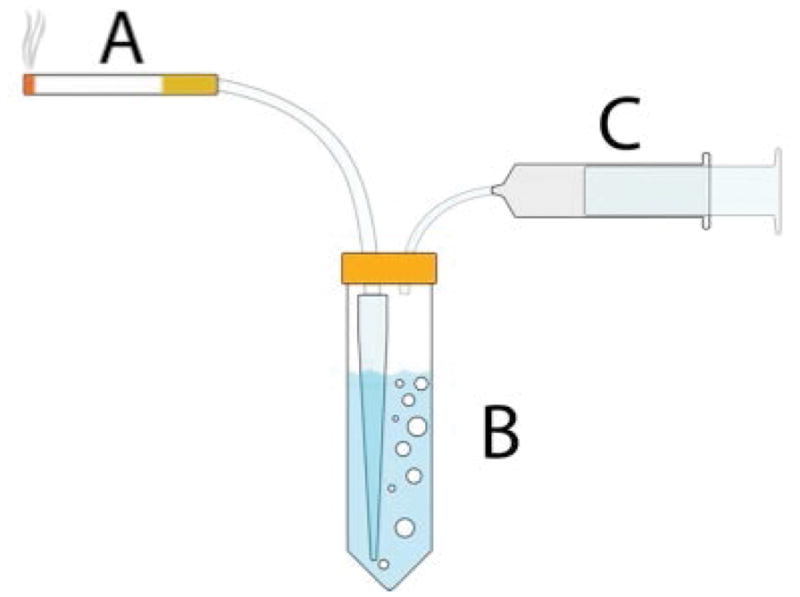
(A, B) Downstream smoke is bubbled through 35 ml of saline solution in a disposable tube. (C). Smoke is drawn by an experimenter-operated syringe over 2 seconds, every 30 seconds.
SUPPORT PROTOCOL 1: Constructing the smoking apparatus
Introductory paragraph
In order to address the gap between clinical and preclinical literature on tobacco dependence, a protocol for aqueous extracts of tobacco smoke has been developed. To make reproducible samples of these extracts, smoking machines have been manufactured. However, these machines are costly and require extensive maintenance. In addition, most smoking machines create a smoke that blows into a chamber instead of collecting the aqueous components to allow for intravenous self-administration and other pharmacological studies. Here we describe a method of manufacturing an inexpensive and easily assembled apparatus to collect the aqueous components of cigarette smoke (see Video for full demonstration of constructing the apparatus).
Materials
50ml polypropylene conical tube cap
10” of large polypropylene tubing (5/16” I.D. x 7/16” O.D., wall thickness 1/16”)
15” of small polypropylene tubing (1/8” I.D. x 1/4” O.D., wall thickness 1/16”)
Plastic barbed straight quick-connect couplers, 3/8 x 1/4” I.D. (Cole-Parmer)
Plastic repair epoxy (fast setting)
Cotton-tipped applicators, 6”, wood shaft
Razor blade
Black Fine Tip Permanent Marker
Ring stand with 2 three-prong clamps
Cyanoacrylate, also known as superglue
Protocol steps: Prepare smoking apparatus
-
On the 50ml conical tube cap, measure and then mark with the permanent marker a dot, 4cm from the center. Make an X on the dot. Cut along the X using a razor blade.
The X should be about the size of the coupler.
Do not use a drill to make the holes as the cap is plastic and you are more likely to break the cap.
-
Insert quick-connect couplers through the X (Figure 2A & B).
Make sure that the flat portion is on top of the cap by inserting the couplers through the top of the cap.
Measure second hole by placing the end of the small tubing on the cap next to the coupler. Mark around the tubing. Cut through the cap along the circle marking with a razor blade.
-
Insert small tubing into hole 2 (Figure 3).
Roughly 0.5” of the small tubing needs to be inserted through the hole.
-
Apply epoxy to apparatus using wood shaft of cotton-tipped applicator
Read directions on epoxy carefully before beginning.
Make sure to seal all holes entirely or apparatus will not work properly.
Epoxy generally sets fast so apply efficiently.
Ensure that couplers are epoxied together.
Allow a full 24hrs for epoxy to set before testing apparatus (Figure 4A & B).
-
Test apparatus by following Basic Protocol 1.
For testing, 1 cigarette should be sufficient to test if all holes are filled.
If cigarette does not smoke properly, fill holes with epoxy, reinforce with superglue, and re-test 24hrs later.
An apparatus does not break but eventually the tubing will turn black. It is suggested to make a new apparatus every 3 months to prevent the nicotine content of CSE from decreasing.
Figure 2.
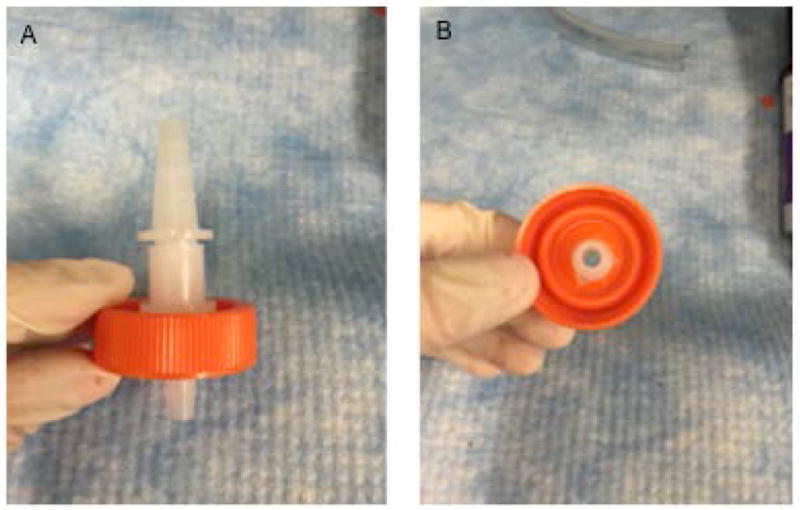
(A) Side and (B) bottom view of cap with quick-connect couplers insert into hole 1.
Figure 3.

Bottom view of cap showing quick-connect coupler and thin tubing inserted.
Figure 4.
(A) Side and (B) bottom view of completed smoking apparatus.
SUPPORT PROTOCOL 2: Analyzing nicotine content
Introductory paragraph
The nicotine content in the CSE samples is analyzed to accurately quantify reproducibility of sample collection. Furthermore, a known nicotine concentration will allow for the examination of the addictive properties of the non-nicotine constituents and nicotine alone. There are a variety of methods for determination of nicotine content in the literature. Here we describe an acid-base extraction of nicotine from CSE combined with gas-chromatography- mass spectrometry for nicotine content determination, a method adapted from Benowitz et al. (1981).
Materials
CSE
(−) - Nicotine hydrogen tartrate salt (Glentham Life Sciences)
2M NaOH solution (Fisher Scientific)
1M HCl (Fisher Scientific)
Dichloromethane (DCM) (Fisher Scientific)
Magnesium sulfate
Glass vials
Vortex mixer
Separation funnel
Gas Chromatography-Mass Spectrophotometer (GC-MS)
Fume hood
Recipe for 2M NaOH and 1M HCl
Dilute 10M NaOH stock solution to 2M NaOH using double distilled water (ddH2O): for a 50 ml total volume of 2M NaOH, mix 10 ml of 10M NaOH with 40 ml double distilled water (ddH2O).
Recipe for 1M HCl
-
Dilute 10M HCl stock solution to 1M HCl using double distilled water (ddH2O): for a 50 ml total volume of 1M HCl, mix 5 ml of 10M HCl with 45 ml double distilled water (ddH2O).
Safety notes: always add acid to water and work in a ventilated fume hood.
Protocol steps: Extract nicotine from samples
-
Pipette 3 ml of CSE into a clean empty glass vial
The vial does not need to be light impenetrable.
-
To 3 ml of CSE, add 1.5 ml of 2M NaOH and 6 ml of DCM. Vortex for 1 min. Collect the organic layer (bottom layer; roughly 7 ml collected).
DCM evaporates very quickly; it is important to always keep all vials capped throughout the extraction process to avoid variations in final volume.
The top layer (aqueous layer) can be disposed in the sink.
-
To organic layer, add 1.5 ml of 1M HCl. Vortex for 1 min. Collect aqueous layer (top layer; roughly 1.5 ml collected).
The bottom layer (organic layer) should be disposed in hazardous chemical waste. Contact your institution’s environmental health and safety department for more information on hazardous chemical waste disposal.
To aqueous layer, add 1.5 ml of 2M NaOH and 1.5 ml of DCM. Vortex for 1 min. Collect organic layer (bottom layer).
-
To organic layer, add about 3 mg of anhydrous magnesium sulfate and let it sit for 5 min. Decant liquid to a clean glass vial and cap tightly.
Samples will be composed of nicotine in DCM. They may be stored in the freezer until GC-MS analysis.
Protocol steps: Run extracts on gas chromatography-mass spectrometry (GC-MS)
Make a 1000 μg/ml solution of nicotine (free base) using saline.
-
Use Support Protocol 1(Steps 1–4)] to extract nicotine from the solution.
Nicotine tartrate salt will not dissolve in DCM so it must be dissolved in saline first, then extracted from the solution into DCM since saline cannot be used on a GC-MS.
-
Create a standard curve by diluting extracted nicotine with DCM.
Useful concentrations would be 1000, 500, 250, 125, 62.5 μg/ml.
-
Run nicotine and CSE samples (extracted using procedures above) on a GC-MS using the 35-10-290 program.
GC-MS used: Finnigan Trace MS with Trace GC 2000 series.
35-10-290 refers to an oven temperature which starts at 35°C and ramps by 10°C/min till it reaches 290°C.
The program used to run samples and analyze data was Xcalibur 1.4.
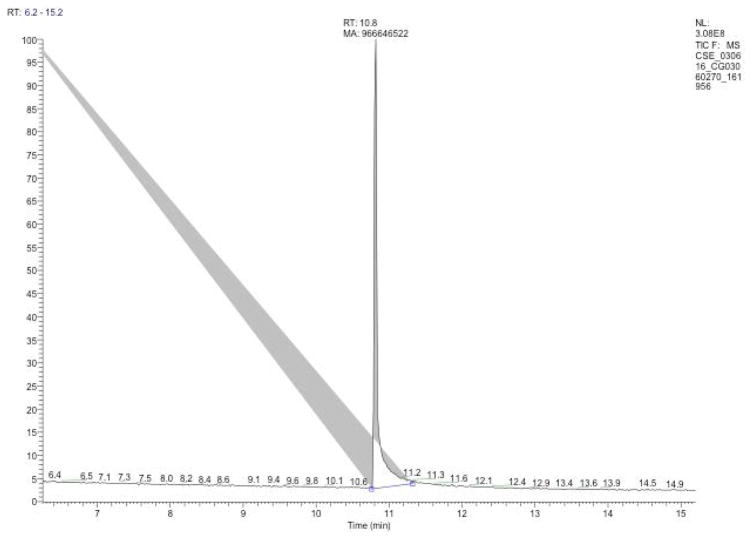
A nicotine peak should be evident around 10 min (Figure 5).
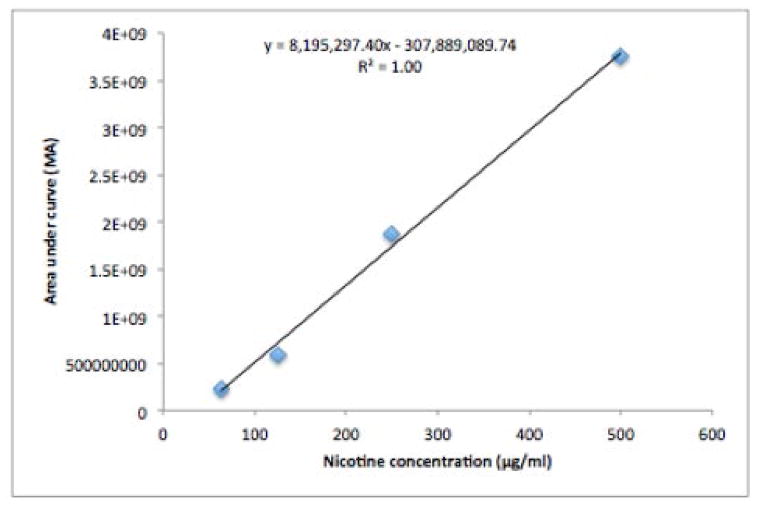
Find the area under the curve for each known concentration of nicotine and plot on a graph, x-axis: concentration, y-axis: area under the curve (AUC; Figure 6).
-
Calculate nicotine concentration of CSE sample by inserting the AUC obtained from the GCMS into the equation calculated from creating the concentration curve. Solve for x, the concentration of nicotine in CSE.
Example: If you were to obtain an AUC of 921,405,520.26 then using the equation in Figure 6 (y=8,195,297.40x-307,889,089.74) you would plug the AUC into y and solve for x. You should obtain a concentration of 150 μg/ml.
Figure 5.
Chromatogram of CSE sample showing a nicotine peak at 10.8 minutes in which the area under the curve (MA) is 966646522 resulting in a nicotine concentration of 156 μg/ml.
Figure 6.
Nicotine concentration curve.
COMMENTARY
Background Information
It has been a challenge to study smoking in preclinical tests due to limitations in methodology that often force investigators to rely on nicotine rather than tobacco smoke. CSE has shown to be a reliable preclinical model of smoking. For instance, CSE inhibits monoamine oxidase activity, as seen in human smokers. Furthermore, adult male rats reliably self-administer low doses of CSE (as in low doses of nicotine in the CSE) that result in blood levels similar to observed in smokers. Animals that self-administer CSE are also more sensitive to stress-induced reinstatement, a major trigger for relapse of smoking in humans (see Costello et. al. (2014) for a thorough review of CSE pharmacology and its comparison to nicotine alone). However, there are limitations to CSE as a preclinical model of smoking. CSE is an aqueous solution of cigarette smoke and, thus, does not contain any non-aqueous constituents. Furthermore, the exact composition of the smoke constituents in CSE is currently unknown. Moreover, the brand of cigarettes used to prepare CSE may result in differing pharmacological profiles. These limitations require further investigation.
Critical Parameters
Maintenance of tubing
Tar in cigarette smoke will clog the inside of the apparatus tubing. Thorough rinsing with warm water is necessary after each use. Replacement of tubing is also recommended every 3–4 months.
If no access to GC-MS
Some commercial facilities can analyze nicotine content from biological fluids. Their method of quantification may work for CSE samples as well. Immediately freeze an aliquot of each CSE sample after production and send it to the facility for analysis on dry ice to avoid degradation of nicotine. A facility that could analyze nicotine content of CSE samples is UCSF Clinical Pharmacology Laboratory.
Troubleshooting
Nicotine content
To ensure consistent results, several batches of CSE should be made and tested before the start of any experiment. Once the technique is perfected and the apparatus is deemed reliable, the content should stay at the consistent range. If the nicotine content in CSE is decreasing, change the tubing of the apparatus. If there is large variation in nicotine content between batches, inspect the apparatus for leaks. Cover openings with epoxy, or construct a new apparatus.
Anticipated Results
The nicotine content of the CSE should be around 150 μg/ml.
CSE is self-administered by rats at 15 μg/kg/infusion nicotine concentration (100 μl intravenous infusions lasting 5.6 sec) (Costello et al 2014). For more on rat operant intravenous self-administration in rats and mice visit Current Protocols in Neuroscience Unit 9.20 (Thomsen and Caine, 2005).
Time Considerations
CSE making: 1hr
CSE apparatus: 30 min
Extraction: 20 min
GC-MS: depends on the machine
SIGNIFICANCE STATEMENT.
Methodological limitations have made it challenging to study tobacco dependence in preclinical models. Here we describe a novel approach of producing an extract of mainstream cigarette smoke in an aqueous medium (Cigarette Smoke Extract; CSE). This solution can be used to study the effects of cigarette smoke constituents on tobacco dependence. CSE is the only model of tobacco dependence composed of mainstream smoke that allows for intravenous self-administration. Most equipment designed to produce a cigarette smoke extract are costly and require frequent maintenance. Here we describe the process of easily assembling an apparatus to make CSE at a significantly lower cost. We also describe a simple method for detecting nicotine concentration in CSE.
Acknowledgments
The authors wish to gratefully acknowledge the support of the National Institute on Drug Abuse, National Institutes of Health (DA 040440) and UC Tobacco Related Diseases Research Program (21RT-0136). All procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
LITERATURE CITED
- Arnold MM, Loughlin SE, Belluzzi JD, Leslie FM. Reinforcing and neural activating effects of norharmane, a non-nicotine tobacco constituent, alone and in combination with nicotine. Neuropharmacology. 2014;85:293–304. doi: 10.1016/j.neuropharm.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30(4):705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med. 1988;319(20):1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US) How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2010. [accessed on: 04.01.16]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK53017. [PubMed] [Google Scholar]
- Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39(8):1843–1851. doi: 10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Mattson C, Lesage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacol Biochem Behav. 2010;96(2):217–227. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004;175(2):134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Manzardo AM, Stein L, Belluzzi JD. Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain Res. 2002;924(1):10–19. doi: 10.1016/s0006-8993(01)03215-2. [DOI] [PubMed] [Google Scholar]
- Mello NK, Newman JL. Discriminative and reinforcing stimulus effects of nicotine, cocaine, and cocaine + nicotine combinations in rhesus monkeys. Exp Clin Psychopharmacol. 2011;19(3):203–214. doi: 10.1037/a0023373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic Intravenous Drug Self-Administration in Rats and Mice. Current Protocols in Neuroscience. 2005;32:9.20:9.20.1–9.20.40. doi: 10.1002/0471142301.ns0920s32. [DOI] [PubMed] [Google Scholar]
- Small E, Shah HP, Davenport JJ, Geier JE, Yavarovich KR, Yamada H, Bruijnzeel AW. Tobacco smoke exposure induces nicotine dependence in rats. Psychopharmacology (Berl) 2010;208(1):143–158. doi: 10.1007/s00213-009-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]