Scheme 2.
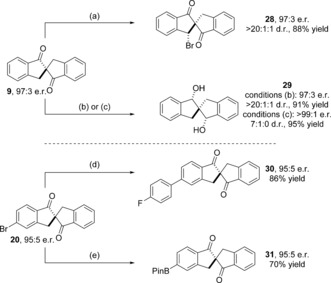
Chemoselective derivatizations of the spirobindanone core. Conditions: a) N‐Bromosuccinimide (1.0 equiv.), AIBN (0.02 equiv.), CCl4, 80 °C. b) DIBALH (1.0 m in THF, 3.0 equiv.), t‐BuLi (1.7 m in pentane, 3.0 equiv.), THF, −78 °C. c) DIBALH (1.0 m in hexanes, 3.3 equiv.), t‐BuLi (1.7 m in pentane, 3 equiv.), THF, −78 °C. d) 4‐F(C6H4)B(OH)2 (1.5 equiv.), Pd(PPh3)4 (2 mol %), Na2CO3, Toluene/H2O (2.3:1, v/v), reflux. e) B2Pin2 (1.2 equiv.), Pd(dppf)Cl2 (5 mol %), KOAc (3.0 equiv.), 1,4‐dioxane, reflux. e.r. determined by chiral stationary phase HPLC; d.r. determined by examination of the crude 1H NMR spectra. Yields refer to isolated materials.
