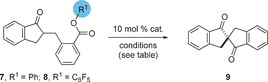
Table 1.
Optimization: cation‐directed enantioselective C‐acylation.[a]
| Entry | R1 | Catalyst | Base | e.r.[b] |
|---|---|---|---|---|
| 1 | Ph | Bu4NBr | K2CO3 (aq.)[c] | 50:50 |
| 2 | Ph | 10 | K2CO3 (aq.)[c] | 56:44 |
| 3 | Ph | 10 | KOH (aq.)[c] | – |
| 4 | Ph | 10 | KOH (s) | 59:41 |
| 5 | Ph | 11 | KOH (s) | 69:31 |
| 6 | Ph | 11 | NaOPh (s) | 51:49 |
| 7 | C6F5 | 11 | KOH (s) | 42:58 |
| 8 | C6F5 | 12 | KOH (s) | 79:21 |
| 9 | C6F5 | 13 | KOH (s) | 25:75 |
| 10 | C6F5 | 14 | KOH (s) | 36:64 |
| 11 | C6F5 | 15 | KOH (s) | 29:71 |
| 12 | C6F5 | 16 | KOH (s) | 17:83 |
| 13 | C6F5 | 17 | KOH (s) | 88:12 |
| 14 | C6F5 | 17 | K3PO4 (s) | 89:11 |
| 15 | C6F5 | 17 | K3PO4 (aq.)[c] | 97:3 |
| ||||
[a] Conditions: substrate 7 or 8 (0.02 mmol), catalyst (10 mol %), solid base (1.0 equiv.), PhMe ([substrate]=0.1 mol dm−3), RT, 48 h. [b] e.r. determined by chiral stationary phase HPLC. [c] base: 50 % aq., w/w, 10.0 equiv.
