ABSTRACT
Introduction: We conducted a study to reveal trends in steroid prescription for Duchenne muscular dystrophy (DMD) patients in Japan. Methods: We asked patients (ages 5–20 years) identified in the patient registry and their clinicians about steroid therapy experiences. Regimen, dose, and starting age were compared among 3 subgroups according to prednisolone initiation year (2000–2004, 2005–2009, and 2010–2013). Results: Among 157 prednisolone users, 4 different regimens were used. Dose frequencies were: every other day (98 patients), daily (44 patients), 10 days on 20 days off (14 patients), and weekly (1 patient). Median starting age was 6 years, and median dose was 0.42 mg/kg/day. There was an increase in daily regimen use from 2005–2009 (n = 9, 16%) to 2010–2013 (n = 33, 36%). Conclusions: This study revealed a transition over time in steroid use from expert opinion to evidence‐based recommendation. Clinical research should be encouraged to optimize medication worldwide. Muscle Nerve 54: 673–680, 2016
Keywords: clinical research, Duchenne muscular dystrophy, expert opinion, guideline, patient registry, regimen, steroid therapy
Abbreviations
- DMD
Duchenne muscular dystrophy
- MD STARnet
Muscular Dystrophy Surveillance Tracking and Research Network
- Remudy
Japanese national registry of muscular dystrophy
- TREAT‐NMD
Translational Research in Europe—Assessment and Treatment of Neuromuscular Diseases
Duchenne muscular dystrophy (DMD) is a rare X‐linked disease that affects 1 in 5,000–6,000 newborn boys.1 The disease follows a progressive course of muscle weakness, including respiratory and cardiac muscles. Affected boys lose the ability to ambulate independently between ages 10 and 12 years.2 Although multiple treatment strategies are under investigation and have shown promise for DMD,3 corticosteroids remain the only drugs with objectively confirmed effects on muscle weakness. Steroid therapy can alter the course of DMD, improve muscle function, and prolong walking ability for 2–5 years.4 The effects of steroids can also be recognized after loss of ambulation in terms of a reduced incidence of scoliosis and slower decline of upper extremity and cardiorespiratory functions.4 However, long‐term, daily use of steroids is associated with various side effects, and several investigations into the optimal timing, dose, and regimen of steroid therapy are ongoing. Alternative dosing regimens, such as weekend dosing (10 mg/kg/week divided over 2 weekend days),5, 6 lower doses,7 and alternate‐day doses8, 9, 10 have been reported, but there is currently no consensus on which offers the most effective regimen with the fewest side effects.11
Steroid therapy for DMD was introduced in Japan in the 1990s.12 Most previous studies elsewhere in the world have used prednisone, which is not available in Japan. Instead, we focus on prednisolone, which was approved for pharmaceutical use in DMD patients in 2013, and practice guidelines were published in 2014.13 We previously reported on the long‐term effects of prednisolone on walking ability.14 In the current study, we carried out a survey to reveal the trends in steroid therapy for DMD in Japan.
MATERIALS AND METHODS
Study Population
A National Registry of Dystrophinopathy (Remudy)15 was established in collaboration with the Translational Research in Europe–Assessment and Treatment of Neuromuscular Diseases (TREAT–NMD) Network of Excellence16 in 2009. As of December 2013, it included 1080 registrants with genetically and clinically diagnosed DMD. Of the 1,080 registrants, we selected 639 patients who were ages 5–20 years (born between 1995 and 2008) (Fig. 1).
Figure 1.
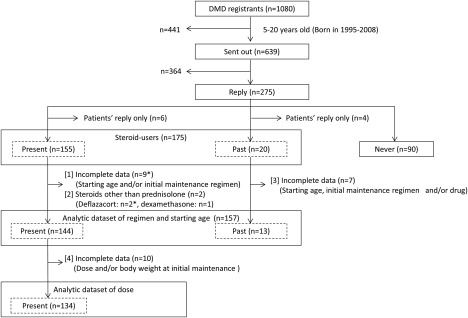
Flow chart of study. *One patient met [1] and [2] of our exclusion criteria. DMD, Duchenne muscular dystrophy; Remudy, Japanese national registry of muscular dystrophy.
Questionnaires
We prepared questionnaires for patients (and/or families) and their clinicians. The patient questionnaire asked if the subject used/had used steroids or not. Steroid users (past or present) were then asked about their experience with steroid therapy, such as subjective efficacy, side effects, and concerns. The clinicians responsible for steroid users were asked about the course of steroid treatment, including steroid name, regimen, dose, starting/stopping age, patient body weight at initiation, reasons for changing dose (increasing and/or decreasing), reasons for stopping steroids, and other drugs used with steroids. Finally, steroid users and their clinicians were asked if they would participate in a future study of steroid therapy, and the clinicians were asked to fill in their name and that of the clinics (Fig. 2).
Figure 2.
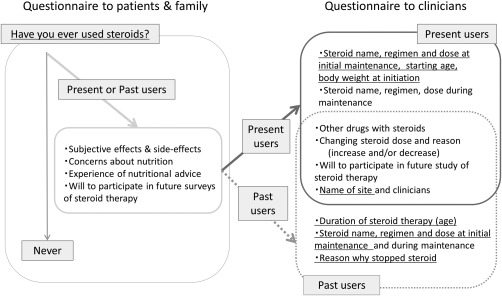
Questionnaires distributed to patients and families, and clinicians who agreed to participate. The underlined questionnaire items were analyzed in this study.
Recruitment
We distributed the combined questionnaire packs (including both the patient and clinician questionnaires) to all 639 patients by mail. When a patient and his family agreed to participate in the study, the clinician questionnaire was distributed to the relevant clinician during the patient's routine clinic visit. We collected replies by mail or electronically using SurveyMonkey® between December 2013 and July 2014.
Statistical Analysis
Categorical variables were expressed as numbers (%), and continuous variables were expressed as median (range: 25th–75th percentile). The dose at steroid initiation was calculated based on the prescribed dose and frequency, and the corresponding body weight, to determine the dose in mg/kg/day:
Outcome
Among the accumulated data, current age and information on steroid initial maintenance (starting age, steroid name, frequency, dose, and body weight) were analyzed. We classified the prednisolone‐users into 3 subgroups based on year of initiation: 2000–2004, 2005–2009, and 2010–2013. We chose the ranges because consensus statements for DMD were published in 2005 and 2010.17, 18 Regimen, age, and dose (mg/kg/day) of prednisolone initial maintenance were compared among the 3 subgroups to determine trends in clinician prescribing behaviors.
Regulatory Issues
Approval for this study was granted by the National Center of Neurology and Psychiatry Ethics Committee. Participation in this survey was voluntary, and we regarded a reply to the questionnaire as agreement to participate. Participants were not compensated for their participation.
RESULTS
Patient Demographics
Of the 639 surveys sent out, 275 recipients responded. Of the 275 replies, 265 were from both patients and clinicians (response rate 41%), and 10 were from patients only. There was a higher response rate from younger patients than older patients (50% of 5‐ to 9‐year‐olds, 40% of 10‐ to 15‐year‐olds, and 34% of 16‐ to 20‐year‐olds). The 265 participants (median age, 11 years) included 155 present‐users (58%, median age, 10 years), 20 past‐users (8%, median age, 16 years), and 90 never‐users (34%, median age, 13 years) (Fig. 1; Table 1). Of the 175 steroid users, 18 (11 present and 7 past) were excluded because of incomplete data (regimen and/or age at initial maintenance) and/or use of steroids other than prednisolone (2 deflazacort, 1 dexamethasone). Finally, data for 157 patients (144 present and 13 past) were analyzed (Fig. 1). Table 2 shows the demographics of prednisolone users classified by year at prednisolone initiation. There were 11 patients (median age 17 years) in 2000–2004, 54 (median age 12 years) in 2005–2009, and 92 (median age 9 years) in 2010–2013.
Table 1.
Patient demographics.a
| Steroid use | ||||
|---|---|---|---|---|
| Present n = 155 | Past n = 20 | Never n = 90 | Total n = 265 | |
| Age (years) | ||||
| Median, range | 10 (5–19) | 16 (10–20) | 13 (5–20) | 11 (5–20) |
| n (%) | n (%) | n (%) | ||
| Birth year | ||||
| 1991–1996 | 8 (19) | 9 (21) | 26 (60) | 43 |
| 1997–2002 | 56 (56) | 9 (9) | 35 (35) | 100 |
| 2003–2008 | 91 (75) | 2 (2) | 29 (24) | 122 |
| Site | ||||
| Neuromuscular centers | 64 (88) | 9 (12) | ‐ | 73 |
| University hospital | 47 (92) | 4 (8) | ‐ | 51 |
| Others | 39 (81) | 7 (19) | ‐ | 46 |
| Unknown | 5 (100) | 0 (0) | ‐ | 5 |
| Area | ||||
| Tohoku, Hokkaido | 13 (93) | 1 (7) | ‐ | 14 |
| Kanto | 76 (89) | 9 (11) | ‐ | 85 |
| Chubu | 23 (82) | 5 (18) | ‐ | 28 |
| Kinki | 19 (86) | 3 (14) | ‐ | 22 |
| Chugoku | 7 (88) | 1 (13) | ‐ | 8 |
| Shikoku | 4 (100) | 0 (0) | ‐ | 4 |
| Kyusyu, Okinawa | 8 (89) | 1 (11) | ‐ | 9 |
| Unknown | 8 (100) | 0 (0) | ‐ | 8 |
We did not ask those who never used steroids to provide medical site and area data.
Table 2.
Demographics of 157 steroid users by year at prednisolone initiation.
| Steroid use, number (%) | Total | Current age Median, range (years) | ||
|---|---|---|---|---|
| Present | Past | |||
| 144 (92) | 13 (8) | 157 (100) | ||
| Year at initiation | ||||
| 2000–2004 | 8 (73) | 3 (27) | 11 (100) | 17 (13–18) |
| 2005–2009 | 46 (85) | 8 (15) | 54 (100) | 12 (9–18) |
| 2010–2013 | 90 (98) | 2 (2) | 92 (100) | 9 (5–14) |
Initial Maintenance Regimen
Four regimens of prednisolone use .were reported: daily in 44 (28%); every other day in 98 (62%); 10 days on 20 days off in 14 (9%); and 2 days/week in 1 (1%) (Fig. 3). There were only 11 patients who initiated in 2000–2004, of whom 2 (18%) used daily, 4 (36%) used every other day, 4 (36%) used 10 days on 20 days off, and 1 (9%) used 2 days/week. Of the 54 patients initiated in 2005–2009, 9 (17%) used daily, 38 (70%) used every other day, and 7 (13%) used 10 days on 20 days off. The largest group included 92 patients initiated in 2010–2013, of whom 33 (36%) used daily, 56 (61%) used every other day, and 3 (3%) used 10 days on 20 days off. Although the every other day regimen was the most frequent by year, the rate of daily use increased from 2005–2009 (n = 9, 17%) to 2010–2013 (n = 33, 36%) (Fig. 3).
Figure 3.
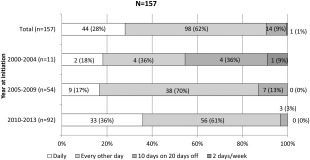
Trends in prednisolone initial maintenance regimens according to year. Every other day regimen was used most frequently. The ratio of daily use increased from 2005–2009 to 2010–2013.
Starting Age
The median prednisolone starting age was 6 years (range: 3–14 years) in steroid users overall, 7 years (range: 4–8 years) in 2000–2004, 6 years (range: 4–14 years) in 2005–2009, and 7 years (range: 3–13 years) in 2010–2013. The data showed bimodal peaks in both 2005–2009 (6 and 10 years) and 2010–2013 (6 and 11 years). Six patients (11%) in 2005–2009 and 11 patients (12%) in 2010–2013 initiated prednisolone after age 10 years (Fig. 4).
Figure 4.
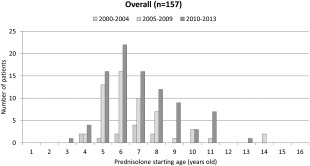
Trends in prednisolone starting age according to year. Bimodal peaks were found in both 2005–2009 (6 and 10 years) and 2010–2013 (6 and 11 years). Six patients in 2005–2009 and 11 patients in 2010–2013 initiated prednisolone after age 10 years.
Initial Maintenance Dose
Of 157 patients, initial maintenance dose was available for 134 present‐users (Fig. 1). The median initial maintenance dose was 0.43 mg/kg/day. According to the year of initiation, the median doses were 0.33 (interquartile range: 0.25–0.49) in 2000–2004, 0.38 (interquartile range: 0.33–0.50) in 2005–2009, and 0.45 (interquartile range: 0.25–0.60) in 2010–2013 (Fig. 5). Six patients who received higher than 0.75 mg/kg/day were detected as outliers (1 in 2000–2004, 2 in 2005–2009, and 3 in 2010–2013). The median doses by regimen (mg/kg/day) were: 0.66, 0.76, and 0.68 in daily (median 0.68); 0.29, 0.38, and 0.31 in every other day (median 0.38); and 0.29, 0.28, and 0.24 in 10 days on 20 days off (median 0.26), respectively. In Table 3, we compared the initial maintenance dose by different starting age, dividing patients into early ambulatory and late ambulatory stages. Among the patients who had started at age 3–7 years, the median dose was 0.33 in 2000–2004, 0.47 in 2005–2009, and 0.47 in 2010–2013. The equivalent doses among patients who had started at 8–13 years were 1.21, 0.30, and 0.33, respectively.
Figure 5.
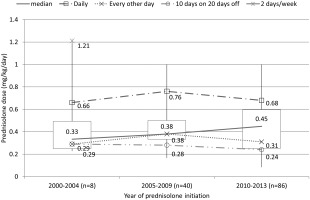
Trends in total prednisolone initial maintenance dose according to year of initiation and regimen. Values are given as medians. The median dose overall by year increased. The median initial maintenance doses for each regimen did not increase by year.
Table 3.
Initial maintenance dose of prednisolone by starting age.
| Starting age | Initiation year | |||
|---|---|---|---|---|
| 2000–2004 | 2005–2009 | 2010–2013 | ||
|
3–7 years (early ambulatory stage) |
n | 7 | 33 | 57 |
|
Dose (mg/kg/day) Median, range |
0.33 (0.23–0.67) | 0.47 (0.25–1.00) | 0.47 (0.12–1.00) | |
|
8–13 years (late ambulatory stage) |
n | 1 | 7 | 29 |
|
Dose (mg/kg/day) Median, range |
1.21 (1.21)a | 0.30 (0.17–0.67) | 0.33 (0.14–0.78) | |
2 days/week regimen user.
Prednisolone Past Users
We received 20 replies from both patients and clinicians who had used prednisolone in the past. Figure 6 shows the profile of 20 past users, including age at starting/stopping prednisolone and the reason for withdrawal. Reasons for withdrawal included side effects (n = 12), loss of ambulation (n = 6), medication in other hospital (n = 1), and unknown (n = 1). Obesity was the most frequent side effect (n = 9) that led to stopping prednisolone.
Figure 6.
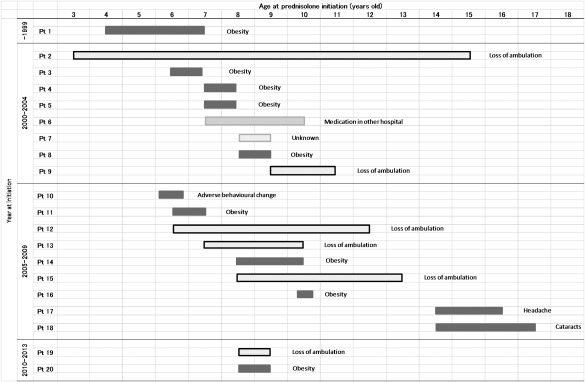
Profile of 20 past users. Duration of steroid‐use and the reason for stopping steroid shown for each past user. Of 20 past users, 7 patients (Pts. 1, 2, 3, 6, 8, 9, and 12) were excluded in the analytic dataset of regimen and age because of missing data. Pt = patient.
DISCUSSION
This study provides evidence for trends in steroid therapy for DMD in Japan. Four regimens of prednisolone use were reported: daily, every other day, 10 days on 20 days off, and 2 days/week. Of these, every other day was the most frequent regimen overall, but daily use increased gradually throughout the study period.
Related studies from western countries have reported that clinical corticosteroid regimens vary11 despite recommendations (Fig. 7).17, 18, 19 According to the survey of 770 DMD caregivers identified through TREAT‐NMD registries, the proportion of patients currently taking glucocorticoids varies significantly between countries and ambulatory classes, with large differences in the prescribed glucocorticoid medications and regimens. Deflazacort was the most commonly used glucocorticoid in Germany, Italy, and the United States. Most U.K. patients were prescribed prednisolone.20 This was also true in Japan, because deflazacort and prednisone were not available in Japan until 2015. Daily dosing predominated in the United States; intermittent regimens (for example, 10 days on 10 days off) were also prescribed in Germany and the United Kingdom, and alternate regimens (for example, every other day) were prescribed in Italy. High‐dose weekend regimens were prescribed exclusively in the United States. In contrast, steroid regimens appear to be relatively consistent within some countries, such as the Netherlands21 and Canada.22 These wide differences reflect the historical background in each area or country, where prescription behaviors have been affected by expert opinion (Fig. 7).
Figure 7.
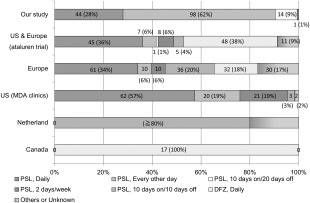
Comparison of steroid regimen between our study and those in other countries. Steroid regimens used have frequently varied between different countries. PSL, prednisolone/prednisone; DFZ, deflazacort; US, United States; MDA, Muscular Dystrophy Association.
Consensus statements17, 18 regard every other day regimens as “less effective, but should be considered when a daily schedule has side effects that are not effectively managed or tolerated”. Prednisolone every other day regimens have been used since steroid therapy for DMD was introduced to Japan, in accordance with a domestic report in 1996.12 The Japanese textbook Child Neurology published in 200823 referred to a prednisolone 0.75 mg/kg/day daily regimen17. However, it also noted that the “optimal dose for Japanese patients was still unclear,” “1 mg/kg every other day regimen or 10 days on 20 days off were used more frequently,” and “the author used daily regimens for patients with low‐risk of obesity.” However, we recently observed some changes, as clinicians' prescribing behaviors changed from expert opinion to evidence‐based recommendation approaches.
Moreover, the development of new medicines for DMD may have encouraged the use of steroid therapy in accordance with recommendations. The median dose of daily prednisone was reported as 0.729 mg/kg/day in MD STARnet,24 and 0.60 mg/kg/day in participants in the Ataluren trial.11 Both of these are similar to that in our study (0.68 mg/kg/day) and are close to the recommended dose (Supplementary Materials, which are available online). In contrast, doses for every other day and 10 days on 20 days off were close to 0.30 mg/kg/day, the minimum effective dose. Increasing daily regimen use seemed to increase the initial maintenance dose overall. In this study, prednisolone dose was lower in 8‐ to 13‐year‐olds than in 3‐ to 7‐year‐olds. We found a median starting age of 6 years, which is similar to that reported in a study of Dutch patients born between 1980 and 2006 (median starting age 6.5 years).21
In a cohort study by MD STARnet, there was a decrease in initiation age from 8.2 years (born 1982–1986) to 7.1 years (born 1997–2001),25 but no such decrease was seen in our study (Supplementary Materials). Approximately 10% of all patients in both 2005–2009 and 2010–2013 in our study initiated prednisolone after age 10 years, which affected the median age. Recently, several long‐term effects of glucocorticoids on the disease after loss of ambulation have been reported, such as slowed progression of scoliosis and stabilization of cardiopulmonary function. However, the effectiveness when initiated in nonambulatory patients is unclear. Therefore, some clinicians may initiate steroids relatively late in the ambulatory phase. Thus, there appears to be a lack of consensus regarding steroid therapy. However, this might be accounted for by the fact that our target population (born in 1995–2008) was younger than in related studies.21, 25 Furthermore, we excluded patients older than 20 years (before 1994), because of the lack of steroid users in the registry. Moreover, we sorted patients by year of initiation to investigate how prescribing trends were affected by the guidelines, while previous research has sorted participants by birth year.25
Among 20 past users, the most common reasons for withdrawal were obesity (60%) and loss of ambulation (30%). In a study by Matthews et al., the most common reasons for withdrawal among 150 patients were weight gain (23.3%), behavioral side effects (19.1%), and loss of ambulation (15.8%).24 While the sample size in our study was smaller, there was a slightly higher rate of obesity than in the related study.
Our study had some limitations. First, questionnaire distribution by means of opt‐in patient registries may have resulted in selection bias. Registries tend to attract patients who are more engaged with the DMD community and who are aware of and more inclined to campaign for best practice care.26 According to a Japanese proceeding, the total number of patients with DMD in Japan as of 2010 was estimated to be 3500, including 1,100 aged 5‐ to 20 years. Based on this assumption, the 639 patients identified would account for 58% of the DMD population aged 5–20, suggesting that our results may not reflect Japanese trends overall. Further selection bias may have been introduced by survey participation, as we had a response rate of 41% (50% in ages 5–9 years, 40% in ages 10–15 years, and 34% in ages 16–20 years).
Steroid therapy effects are more recognized than before in Japan. As such, younger patients might be more interested in their treatment. Moreover, recall bias may be increased in patients who initiated steroid therapy earlier. Also, the low response rate in older patients might not only make the results less reliable but also affect the results compared by initiation year. Furthermore, age at initiation was only recorded to the nearest year to simplify the responses. Nevertheless, the results suggest that use of a daily steroid regimen increased significantly, irrespective of any bias.
This nationwide survey, carried out by means of Remudy, is a report of steroid therapy for patients with DMD in clinical practice in an Asian country. Li et al.27 described a database of DMD and Becker muscular dystrophy patients in East China, and reported the distribution of steroid use in registrants with DMD. Few Asian studies have discussed steroid regimen, dose, and initiation age in detail. We previously reported a large‐scale retrospective study of the long‐term effects of prednisolone on walking ability using the same registry database. However, this previous study did not analyze regimen, dose, or starting age, because these items had not been included in the database. We address these variables in the present study. Our studies emphasize the importance of patient registries in contributing to developments in existing care and treatment and as a resource for future trials databases. In this study, we did not focus on patients' subjective report of effectiveness, side effects, and concerns about steroid therapy, instead we report on clinicians' prescription behavior. Further prospective studies are needed to assess the effects and tolerability of steroid therapy by including more parameters with appropriate outcome measures.
Prednisolone has been approved for pharmaceutical use in DMD patients since 2013, and domestic guidelines for DMD were published in 2014.13 These innovations should raise the awareness of steroid therapy among clinicians, patients, and their families nationwide. This study analyzed prescribing behaviors before publication of the domestic guideline, and the surveys should be repeated to monitor trends in steroid therapy and to consider adherence to the guidelines, as noted by Fox et al.25
Prospective studies comparing the effect and safety of frequent and recommended regimens have recently been conducted in western countries. Escolar et al.6 reported a randomized blinded clinical trial comparing high‐dose weekend prednisone (10 mg/kg/week) with the recommendation (prednisone 0.75 mg/kg/day) and concluded that the high‐dose weekend regimen was as effective and safe as the daily regimen. Ricotti et al.28 performed a 4‐year prospective longitudinal observational study comparing prednisolone 10 days on, 10 days off with prednisolone daily. They found no significant difference in the median age at loss of ambulation, though there were differences in the severity of side effects. A 5‐year international, multicenter, randomized, double‐blind, controlled clinical trial has been started (FOR‐DMD) to evaluate efficacy and safety among the 3 most frequent regimens in western countries: prednisone 0.75 mg/kg/day daily, deflazacort 0.9 mg/kg/day daily, and prednisone 0.75 mg/kg/day for 10 days on, 10 days off.
The effects of prednisolone every other day regimens have been reported in 2 Japanese studies. Sato et al.29 evaluated the effects of prednisolone on intellectual ability and motor function in 20 patients using prednisolone 0.75 mg/kg every other day (0.12–0.5 mg/kg/day) for 2 years in comparison with 9 controls. Intelligence quotient scores improved, and motor function was preserved in the treated group. Ishigaki et al.30 compared longitudinal clinical data between 7 steroid treated (prednisolone 0.5 mg/kg, every other day) and 10 nontreated patients over age 15 years. Ages at loss of ambulation and initiation of noninvasive ventilation did not differ. There was an earlier onset of cardiomyopathy in 2 treated patients than in the nontreated, and the ratio of cardiomyopathy development differed significantly (29% vs. 90%; P = 0.018). We need to assess efficacy and safety between daily and every other day regimens, as evidence for this is limited worldwide8.
Clinical research should be encouraged in Asian and western countries to optimize steroid therapy worldwide. As demonstrated by Ricotti et al.,28 it is important to collect longitudinal data on patients treated according to standardized protocols and to identify the benefits and side effect profiles of different treatment regimens. This will provide evidence for informed choice for patients, families, and clinicians not only in Japan but also worldwide. We also need to assess the efficacy and safety profile of starting and continuing glucocorticoid administration in nonambulant patients. The implementation of multidisciplinary interventions, particularly respiratory management, means that the life expectancy of DMD patients has shifted to the third/fourth decades.31 It is, therefore, necessary to carry out longitudinal analyses to evaluate survival and quality of life in DMD patients and to determine the optimal treatment protocol in terms of continuing steroid therapy beyond loss of ambulation.
It is hoped that new drugs to cure DMD will be developed in the near future. In the meantime, corticosteroids are the only available medicines that have a proven effect on muscle weakness in boys with DMD. Our study showed some changes in clinician prednisolone prescribing behaviors for DMD from expert opinion to an evidence‐based recommendation approach. However, further studies are needed in Japan, as in western countries, to provide evidence to allow optimization of steroid therapy for DMD worldwide.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Tables.
ACKNOWLEDGMENTS
The authors thank the patients, families, and clinicians for participating in our survey and co‐operating with the Japanese national registry of muscular dystrophy (Remudy). We also thank Ms. Mikiko Shigemori and the Remudy office secretarial staff for help with the survey. The authors thank Edanz (http://www.edanzediting.co.jp/) for English language editing. Remudy is operated in collaboration with TREAT‐NMD alliance. This study was supported by an Intramural Research Grant (26‐6 and 26‐7) for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry. Authors' Contributions: Fumi Takeuchi: initial study concept, study design, acquisition of data, analysis, interpretation and preparation of the first/second draft. Hirofumi Komaki: study design, interpretation and preparation of the first/second draft. Harumasa Nakamura: initial study concept, study design, interpretation and preparation of the second draft. Naohiro Yonemoto: initial study concept, study design, interpretation and preparation of the first/second draft. Kousuke Kashiwabara: analysis, interpretation and preparation of the first/second draft. En Kimura: initial study concept, study design, interpretation, and preparation of the second draft. Shin'ichi Takeda: study supervision. The authors declare that they have no conflicts of interest.
REFERENCES
- 1. Mendell JR, Shilling C, Leslie ND, Flanigan KM, al‐Dahhak R, Gastier‐Foster J, et al Evidence‐based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 2012;71:304–313. [DOI] [PubMed] [Google Scholar]
- 2. Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Florence J, et al Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology 1989;39:475–481. [DOI] [PubMed] [Google Scholar]
- 3. Leung DG, Wagner KR. Therapeutic advances in muscular dystrophy. Ann Neurol 2013;74:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moxley RT III, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in natural history of Duchenne muscular dystrophy with long‐term corticosteroid treatment: implications for management. J Child Neurol 2010;25:1116–1129. [DOI] [PubMed] [Google Scholar]
- 5. Connolly AM, Schierbecker J, Renna R, Florence J. High dose weekly oral prednisone improves strength in boys with Duchenne muscular dystrophy. Neuromuscul Disord 2002;12:917–925. [DOI] [PubMed] [Google Scholar]
- 6. Escolar DM, Hache LP, Clemens PR, Cnaan A, McDonald CM, Viswanathan V, et al Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology 2011;77:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brooke MH. Clinical investigation of Duchenne Muscular Dystrophy. Arch Neurol 1987;44:812. [DOI] [PubMed] [Google Scholar]
- 8. Fenichel GM, Mendell JR, Moxley RT III, Griggs RC, Brooke MH, Miller JP, et al A comparison of daily and alternate‐day prednisone therapy in the treatment of Duchenne muscular dystrophy. Arch Neurol 1991;48:575–579. [DOI] [PubMed] [Google Scholar]
- 9. Beenakker EA, Fock JM, Van Tol MJ, Maurits NM, Koopman HM, Brouwer OF, et al Intermittent prednisone therapy in Duchenne muscular dystrophy: a randomized controlled trial. Arch Neurol 2005;62:128–132. [DOI] [PubMed] [Google Scholar]
- 10. Sansome A, Royston P, Dubowitz V. Steroids in Duchenne muscular dystrophy; pilot study of a new low‐dosage schedule. Neuromuscul Disord 1993;3:567–569. [DOI] [PubMed] [Google Scholar]
- 11. Griggs RC, Herr BE, Reha A, Elfring G, Atkinson L, Cwik V, et al Corticosteroids in Duchenne muscular dystrophy: major variations in practice. Muscle Nerve 2013;48:27–31. [DOI] [PubMed] [Google Scholar]
- 12. Kang J. Glucocorticoid therapy in Duchenne muscular dystrophy. [Article in Japanese]. Rinsho Shinkeigaku 1996;36. [PubMed] [Google Scholar]
- 13.Japanese Society of Neurology, Japanese Society of Child Neurology, National Center of Neurology and Psychiatry, 2014. Nankodo: Clinical care and treatment guideline for Duchenne Muscular Dystrophy 2014. Available at https://www.neurology-jp.org/guidelinem/dmd.html. Accessed 2016 March 3.
- 14. Takeuchi F, Yonemoto N, Nakamura H, Shimizu R, Komaki H, Mori‐Yoshimura M, et al Prednisolone improves walking in Japanese Duchenne muscular dystrophy patients. J Neurol 2013;260:3023–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamura H, Kimura E, Mori‐Yoshimura M, Komaki H, Matsuda Y, Goto K, et al Characteristics of Japanese Duchenne and Becker muscular dystrophy patients in a novel Japanese national registry of muscular dystrophy (Remudy). Orphanet J Rare Dis 2013;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bladen CL, Rafferty K, Straub V, Monges S, Moresco A, Dawkins H, et al The TREAT‐NMD Duchenne muscular dystrophy registries: conception, design, and utilization by industry and academia. Hum Mutat 2013;34:1449–1457. [DOI] [PubMed] [Google Scholar]
- 17. Moxley RT III, Ashwal S, Pandya S, Connolly A, Florence J, Mathews K, et al Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2005;64:13–20. [DOI] [PubMed] [Google Scholar]
- 18. Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010;9:77–93. [DOI] [PubMed] [Google Scholar]
- 19. Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol 2010;9:177–189. [DOI] [PubMed] [Google Scholar]
- 20. Landfeldt E, Lindgren P, Bell CF, Schmitt C, Guglieri M, Straub V, et al Compliance to care guidelines for Duchenne Muscular Dystrophy. J Neuromuscul Dis 2015;2:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van den Bergen JC, Ginjaar HB, van Essen AJ, Pangalila R, de Groot IJM, Wijkstra PJ, et al Forty‐five years of Duchenne Muscular Dystrophy in The Netherlands. J Neuromuscul Dis 2014;1:99–109. [PubMed] [Google Scholar]
- 22. McMillan HJ, Campbell C, Mah JK; Canadian Paediatric Neuromuscular Group . Duchenne muscular dystrophy: Canadian paediatric neuromuscular physicians survey. Can J Neurol Sci 2010;37:195–205. [DOI] [PubMed] [Google Scholar]
- 23. Komaki H. Muscular Dystrophy. In: Arima M editors. Child Neurology. Tokyo: SHINDAN TO CHIRYO SHA; 2008. p371–377. [Google Scholar]
- 24. Matthews DJ, James KA, Miller LA, Pandya S, Campbell KA, Ciafaloni E, et al Use of corticosteroids in a population‐based cohort of boys with duchenne and becker muscular dystrophy. J Child Neurol 2010;25:1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fox DJ, Kumar A, West NA, DiRienzo AG, James KA, Oleszek J, et al Trends with corticosteroid use in males with Duchenne muscular dystrophy born 1982–2001. J Child Neurol 2015;30:21–26. [DOI] [PubMed] [Google Scholar]
- 26. Rodger S, Woods KL, Bladen CL, Stringer A, Vry J, Gramsch K, et al Adult care for Duchenne muscular dystrophy in the UK. J Neurol 2014;26:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X, Zhao L, Zhou S, Hu C, Shi Y, Shi W, et al A comprehensive database of Duchenne and Becker muscular dystrophy patients (0–18 years old) in East China. Orphanet J Rare Dis 2015;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ricotti V, Ridout DA, Scott E, Quinlivan R, Robb SA, Manzur AY, et al Long‐term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry 2013;84:698–705. [DOI] [PubMed] [Google Scholar]
- 29. Sato Y, Yamauchi A, Urano M, Kondo E, Saito K. Corticosteroid therapy for duchenne muscular dystrophy: improvement of psychomotor function. Pediatr Neurol 2014;50:31–37. [DOI] [PubMed] [Google Scholar]
- 30. Ishigaki K. Long‐term and low‐dose steroid therapy for cardiomyopathy in Duchenne muscular dystrophy patients. J Tokyo Wom Med Univ 2013;83(Extra):E14–E19. [Google Scholar]
- 31. Kohler M, Clarenbach CF, Bahler C, Brack T, Russi EW, Bloch KE. Disability and survival in Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry 2009;80:320–325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Tables.


