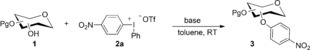
Table 1.
Screening of the arylation of secondary hydroxy groups.[a]
| Entry | 1 | Base | t | 3 | Yield [%][a] |
|---|---|---|---|---|---|
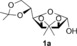
| 1[b] |
|
tBuOK | 20 min | 3 a | 95 |
| 2 | KOH | 3 h | 96 | ||
| 3 | NaOH | 3 h | 95 | ||
| 4 | NaOH(aq) [c] | 3 h | 93 | ||
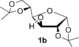
| 5 |
|
NaOH | 2 h | 3 b | quant |
| 6[b] | tBuOK | 40 min | 92 | ||
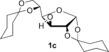
| 7 |
|
NaOH | 5 h | 3 c | 89 |
| 8[b] | tBuOK | 10 min | 97 | ||
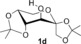
| 9 |
|
NaOH | 3 h | 3 d | 99 |
| 10[b] | tBuOK | 10 min | 99 |
[a] Reaction conditions: sugar 1 (0.2 mmol), salt 2 a (1.5 equiv) and base (1.5 equiv), toluene (1 mL), RT. Yield of isolated product. [b] 0.4 mmol scale. [c] 0.3 mmol NaOH in 100 μL water. Tf=trifluoromethanesulfonyl.
