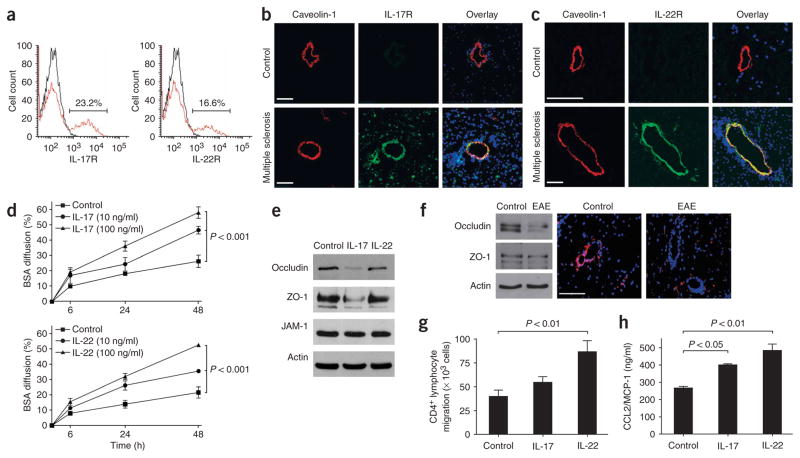
Figure 2.
IL-17 and IL-22 receptors are expressed on human brain endothelium, and their activation permeabilizes the BBB. (a) Unactivated human BBB-ECs grown in primary culture were stained for IL-17R and IL-22R, revealing their expression on the surface of 23.2% and 16.6% of BBB-ECs, respectively. (b) Human CNS postmortem material from unaffected individuals (control, non-inflamed; above) and heavily infiltrated CNS material from individuals with multiple sclerosis (below) were immunostained for IL-17R (green), caveolin-1 (red) and nuclear stain TO-PRO3 (blue). Confocal microscopy imaging confirmed the expression of IL-17R on caveolin-1+ endothelium in inflamed CNS material. IL-17R expression was undetectable in control CNS material. Bar, 75 μm. (c) Similarly, IL-22R staining was observed on endothelial cells in multiple sclerosis lesions, but not in controls. (d) Human BBB-ECs were grown in Boyden chambers and treated with IL-17 (top) or IL-22 (bottom). Permeability of the monolayers was monitored with fluorescent BSA, showing that BBB-EC monolayer permeability increased after treatment with either IL-17 or IL-22. (e) Western blot for the tight-junction proteins occludin, ZO-1 and junction adhesion molecule (JAM)-1 from human BBB-ECs revealed disruption of occludin and ZO-1 by IL-17 (100 ng/ml, 18 h). (f) Western blot for tight-junction proteins in spinal cord homogenates of MOG35–55-immunized EAE mice revealed a similar reduction in occludin and ZO-1. In situ immunostaining for ZO-1 (red) and nuclear stain TO-PRO3 (blue) in normal-appearing cerebellar white matter (control) and in infiltrated and demyelinated cerebellar lesions from C57BL/6 mice immunized with MOG35–55 (EAE, grade 4). Confocal microscopy imaging confirmed disruption of ZO-1 around infiltrated vessels. Bar, 75 μm. (g) Freshly isolated peripheral blood human CD4+ lymphocytes were allowed to migrate for 18 h across IL-17– (100 ng/ml) or IL-22–treated (100 ng/ml) human BBB-ECs. Both cytokines promoted migration of human ex vivo CD4+ lymphocytes across human BBB-ECs, as compared to control. (h) CCL2 (or MCP-1) secretion by human BBB-ECs was assessed by ELISA in untreated and IL-17– or IL-22–treated cultures (100 ng/ml, 18 h). Both IL-17 and IL-22 upregulate CCL2 secretion by human BBB-ECs. All data shown represent the mean ± s.e.m. from three independent experiments performed in triplicate.