Abstract
Unlike mammals, zebrafish efficiently regenerate functional nervous system tissue after major spinal cord injury. Whereas glial scarring presents a roadblock for mammalian spinal cord repair, glial cells in zebrafish form a bridge across severed spinal cord tissue and facilitate regeneration, a relatively unexplored process. Here, we performed a genome-wide profiling screen for secreted factors that are upregulated during zebrafish spinal cord regeneration. We find that connective tissue growth factor a (ctgfa) is induced in and around glial cells that participate in initial bridging events. Mutations in ctgfa disrupt spinal cord repair, while transgenic ctgfa overexpression and local human CTGF recombinant protein delivery accelerate bridging and functional regeneration. Our study reveals that CTGF is necessary and sufficient to stimulate glial bridging and natural spinal cord regeneration.
The spinal cord of adult zebrafish recovers spontaneously after injury (Fig. 1A and Fig. S1 A, B). Efficient axon growth, adult neurogenesis, and absence of glial scarring distinguish this injury response from that in mammals (1, 2). Following initial inflammation, ependymal cells proliferate and glia form a bridge that is thought to provide a scaffold for axonal growth (3). The severed cord reconnects, and new neuronal connections lead to functional recovery (Fig. 1A)(4). Here, we analyzed extracellular factors upregulated in the regenerating zebrafish spinal cord.
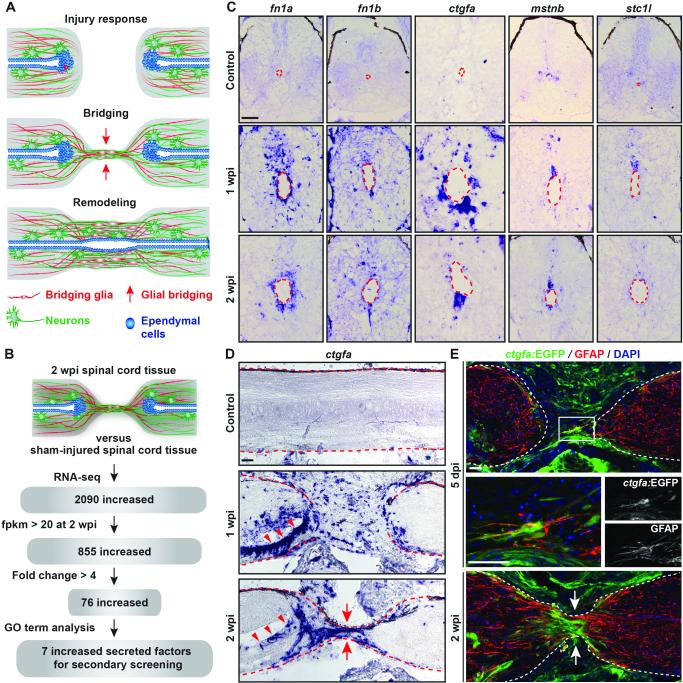
Fig. 1. Identification of ctgfa from a screen for regulators of spinal cord regeneration.
(A) Schematic of the multi-step process of spinal cord regeneration in zebrafish. (B) A screen for secreted factors expressed during spinal cord regeneration. (C) In situ hybridization on spinal cord cross sections at 1 and 2 weeks post-injury (wpi) and in uninjured control tissue. Sections proximal to the lesion from the rostral side are shown, and dashed lines delineate the central canals. The canal dilates after injury. (D) ctgfa in situ hybridization on longitudinal spinal cord sections at 1 and 2 wpi, and in uninjured control tissue. (E) ctgfa:EGFP reporter expression and GFAP immunohistochemistry during early bridging events at 5 days post-injury (dpi; Top) and after bridge formation at 2 wpi (Bottom). (Middle) High-magnification view of boxed area in top panel. Dashed lines delineate spinal cord edges, arrows point to sites of bridging, and arrowheads point to ventral ependymal cells. Scale bars, 50 μm.
To identify potential secreted, pro-regenerative signaling molecules, we screened zebrafish transcriptomes for genes induced after spinal cord injury (Fig. 1B and Table S1). Our screen identified 7 genes encoding secreted, extracellular proteins, including fibronectin 1 a (fn1a), previously implicated in axon growth promotion (5, 6). Transcripts for fn1a, fn1b, connective tissue growth factor a (ctgfa), myostatin b (mstnb), and stanniocalcin 1 like (stc1l) increased in ependymal cells at 1 and 2 wpi (Fig. 1C and Fig. S1C). Whereas fn1a was expressed around the entirety of the central canal at 2 wpi, mstnb and ctgfa were predominantly expressed in the dorsal and ventral ependyma, respectively (Fig. 1C).
CTGF is a matricellular, multifunctional protein that can influence the activity of multiple major signaling pathways, affecting cell adhesion, migration, proliferation, and differentiation. CTGF expression increases after central nervous system (CNS) trauma in rodents, but its function after spinal cord injury has not been elucidated (7-9). As ctgfa is induced upon spinal cord injury in zebrafish, we hypothesized that it may have pro-regenerative roles. We analyzed ctgfa expression along the rostrocaudal spinal cord axis (Fig. 1D). At 1 wpi, ctgfa transcription was induced in multiple cell types across the lesion site and in ependymal cells at the central canal near the lesion. At this timepoint, we detected strongest ctgfa expression in the ventral ependyma. By 2 wpi, ctgfa expression localized to ventral ependymal cells and marked the cellular bridge that had formed at the lesion site. Expression declined beyond 3 wpi (Fig. S2A). Thus, ctgfa expression correlates with formation of the glial bridge.
To identify the cell populations that express ctgfa during spinal cord repair, we generated transgenic reporter zebrafish with a 5.5 kb genomic sequence upstream of the ctgfa translational start site fused to a EGFP reporter cassette. ctgfa:EGFP fluorescence resembled endogenous ctgfa mRNA expression in spinal cord tissue at 2 wpi (Fig. S2 B, C). As early as 5 days post-injury (dpi), domains of ctgfa:EGFP and GFAP, a marker of multiple glial cells in the CNS, overlapped within a subpopulation of glial cells at the injury site (Fig. 1E). We interpret these to be bridging cells. Similarly, ctgfa:EGFP colocalized with the GFAP+ bridge at 2 wpi (Fig. 1E). Comparison of ctgfa:EGFP and GFAP expression on serial cross sections revealed that ~97% of GFAP+ bridging glia at the lesion core were also EGFP+ at 1 wpi (Fig. S3). Further away from the lesion, ctgfa:EGFP was mainly present in ventral ependymal cells (Fig. S3). We also detected ctgfa:EGFP in skeletal muscle, bone cells, and reactive fibroblast-like cells around the lesion site. ctgfa-driven expression suggested delineation of “pioneer” bridging glia.
To determine if ctgfa is required for spinal cord regeneration, we generated a ctgfa mutant allele (ctgfabns50; referred to as ctgfa−) that harbors a frameshift-causing, 7-nt deletion within the third exon of the ctgfa locus (Fig. S4 A-C). ctgfa−/− animals are adult viable and appear to have unaffected motor function capacity (10) (Fig. S4D). However, ctgfa−/− animals showed diminished swim capacity after spinal cord injury, with no significant functional recovery between 2 and 6 wpi (Fig. 2A). Heterozygous (ctgfa+/−) animals showed partial recovery of swim capacity by 6 wpi (Fig. 2A). At 4 wpi, anterograde axon tracing indicated that axon regeneration across the lesion site was reduced by ~25% in ctgfa+/− and ~60% in ctgfa−/− spinal cords proximal to lesion, and by ~40% in ctgfa+/− and ~80% in ctgfa−/− cords distally (Fig. 2B). Thus ctgfa is required for spinal cord regeneration.
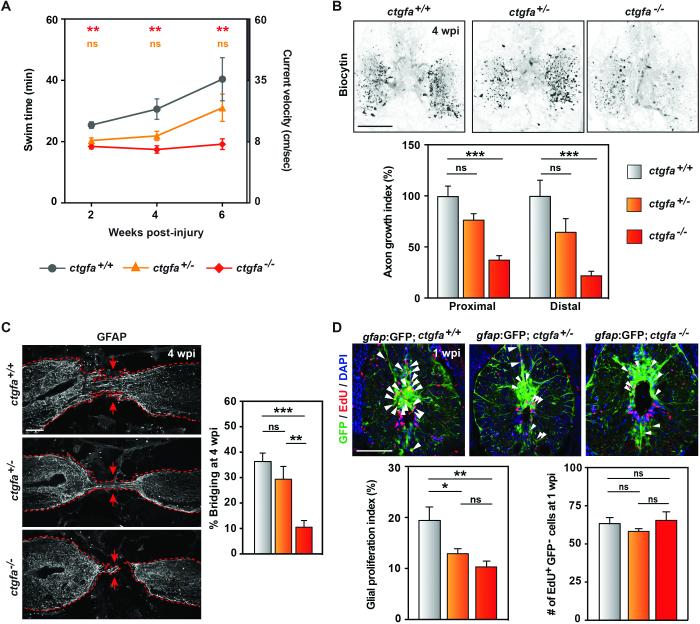
Fig. 2. ctgfa is necessary for glial bridging and spinal cord regeneration.
(A) Swim assays assessed animals’ capacity to swim against increasing water current inside an enclosed swim tunnel. Seven wild-type (ctgfa+/+), 10 ctgfa heterozygous (ctgfa+/−) and 10 mutant (ctgfa−/−) clutchmates were assayed at 2, 4, and 6 wpi. Statistical analyses of swim times are shown for ctgfa−/− (red) and ctgfa+/− (orange) compared to wild-types. Recovery of ctgfa−/− animals was not significant between 2 and 6 wpi. (B) Anterograde axon tracing in ctgfa mutant animals at 4 wpi. For quantification of axon growth at areas proximal (shown in images) and distal to the lesion core, 16 wild-type, 17 ctgfa+/−, and 20 ctgfa−/− zebrafish from 2 independent experiments were used. (C) GFAP immunohistochemistry in ctgfa mutant spinal cords at 4 wpi. Percent bridging was quantified for 10 wild-type, 9 ctgfa+/− and 10 ctgfa−/− clutchmates from 3 independent experiments. Dashed lines delineate glial GFAP staining, and arrows point to sites of bridging. (D) Glial cell proliferation in wild-type, ctgfa+/−, and ctgfa−/− spinal cords at 1 wpi. For quantification of glial proliferation indices (left) and number of EdU-positive gfap:GFP-negative cells (right), 10 wild-type, 12 ctgfa+/−, and 15 ctgfa−/− animals from 2 independent experiments were used. For statistical analyses, (*), (**) and (***) represent P-values of <0.05, <0.01, and <0.001, respectively; while (ns) indicates P-values > 0.05. Scale bars, 100 μm.
Deficits in glial bridging might underlie the defects in spinal cord regeneration displayed by ctgfa mutants. By GFAP and acetylated-α-tubulin immunohistochemistry, we observed robust glial bridges in wild-type and ctgfa+/− animals at 4 wpi (Fig. 2C and Fig. S5A). However, ctgfa−/− animals displayed ~71% less bridging than wild-type clutchmates (Fig. 2C and Fig. S5A). At 2 wpi, glial cells within ctgfa−/− injury sites often failed to extend projections into the lesion (Fig. S5B). Using EdU incorporation assays, ctgfa−/− zebrafish displayed a ~48% reduction in glial cell proliferation at 1 and 2 wpi (Fig. 2D and Fig. S6). EdU+GFAP− cell numbers were comparable in wild-type, ctgfa+/− and ctgfa−/− tissues at 1 wpi (Fig. 2D), suggesting that the effects of ctgfa mutations were preferential to glial cells. Thus, ctgfa is required for the changes in proliferation and morphology of glial cells during spinal cord regeneration.
To examine effects of excess ctgfa on spinal cord regeneration, we generated and injured transgenic fish that express full-length ctgfa under control of a heat-inducible promoter (hsp70:ctgfa-FL) (Fig. S7). Recovery of swim capacity was improved in ctgfa-overexpressing animals given daily heat shocks and assessed at 2, 4, and 6 wpi (Fig. 3A). Swim capacity was comparable between sham-injured hsp70:ctgfa-FL and wild-type clutchmates at 2 and 6 weeks post-heat shock (Fig. 3A), indicating contextual effects of ctgfa overexpression. Histology indicated increased bridging and axon regeneration in ctgfa-overexpressing fish compared to controls at 2 and 4 wpi, respectively (Fig. 3 B, C and Fig. S8A). Thus, whole-animal ctgfa overexpression promotes regeneration after spinal cord injury.
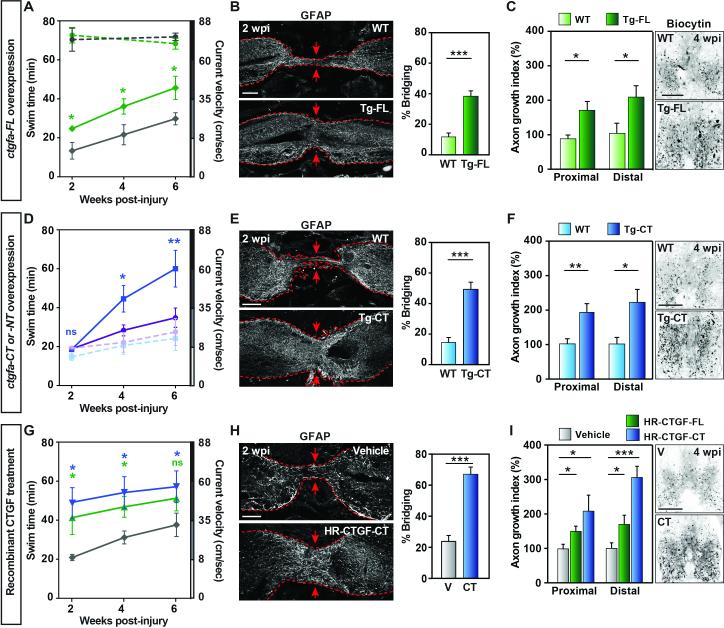
Fig. 3. ctgfa promotes glial bridging and spinal cord regeneration.
(A) Swim assays determined motor function recovery of 10 hsp70:ctgfa-FL (green) and 10 wild-type (gray) clutchmates at 2, 4, and 6 wpi. For sham controls, 8 ctgfa-FL-overexpressing (dashed green) and 7 wild-types (dashed gray) were analyzed. Statistical analyses of swim times are shown for injured ctgfa-FL (green) compared to wild-types. (B) GFAP immunohistochemistry was used to quantify glial bridging at 2 wpi in 18 ctgfa-FL-overexpressing and 16 wild-types from 3 independent experiments. (C) Anterograde axon tracing at 4 wpi after ctgfa-FL overexpression. Quantification at areas proximal (shown in images) and distal to the lesion core represents 12 ctgfa-FL-overexpressing and 10 wild-type zebrafish from 2 independent experiments. (D) Swim assays for 8 ctgfa-CT- overexpressing (blue), 10 ctgfa-NT-overexpressing (violet), and 9 wild-type clutchmate animals (wild-type controls for -CT in dashed blue and for -NT in dashed violet). Statistical analyses of swim times are shown for ctgfa-CT (blue) compared to wild-types. (E) Glial bridging at 2 wpi in 19 ctgfa-CT-overexpressing and 20 wild-type animals from 2 independent experiments. (F) Anterograde axon tracing at 4 wpi after ctgfa-CT overexpression. Quantification represents 16 ctgfa-CT-overexpressing and 16 wild-type animals from 2 independent experiments. (G) Swim capacity was assessed for 9 vehicle- (gray), 8 HR-CTGFFL- (green) and 9 HR-CTGF-CT- (blue) treated animals. Statistical analyses are shown for HRCTGF-FL (green) and HR-CTGF-CT (blue) treatments compared to vehicle controls. (H) Glial bridging at 2 wpi in 18 HR-CTGF-CT-treated and 15 vehicle-treated animals from 3 independent experiments. (I) Anterograde axon tracing at 4 wpi after HR-CTGF-CT treatment. Quantification represents 18 vehicle-, 16 HR-CTGF-FL- and 14 HR-CTGF-CT-treated animals from 2 independent experiments. For histology in (B, E, H), dashed lines delineate glial GFAP staining, and arrows point to sites of bridging. For statistical analyses, (*), (**), and (***) represent P-values of <0.05, <0.01, and <0.001 respectively. Scale bars, 100 μm.
CTGF harbors 4 protein interaction domains and a protease domain that self-cleaves CTGF into pro-fibrotic N-terminal and proliferative C-terminal peptides (11, 12). To determine the active portion of zebrafish Ctgfa during spinal cord regeneration, we created transgenic fish with heat-inducible expression of either N-terminal or C-terminal ctgfa fragments (hsp70:ctgfa-NT and hsp70:ctgfa-CT) (Fig. S7A). Only ctgfa-CT overexpression recapitulated the pro-regenerative effects of ctgfa-FL (Fig. 3, D-F). By 6 wpi, swim capacity was markedly increased in ctgfa-CT-overexpressing animals compared to ctgfa-NT-overexpressing or wild-type clutchmates (Fig. 3D). Anatomically, ctgfa-CT overexpression resulted in over 3-fold and over 2-fold increased glial bridging and axon growth at 2 and 4 wpi, respectively (Fig. 3 E, F and Fig. S8B). These experiments indicate that the pro-regenerative activity of Ctgfa maps to its C-terminal domains.
To examine whether effects of Ctgfa augmentation could be reproduced by localized delivery into the spinal cord lesion site, we injured wild-type animals and applied human recombinant CTGF (HR-CTGF-FL and HR-CTGF-CT) peptides adjacent to the lesion site at 5 and 10 days post-injury (dpi) using a gelfoam sponge. We then assessed regeneration at 2 and 4 wpi, corresponding to 9 and 23 days post-treatment. Human CTGF and zebrafish Ctgfa are 81% identical and 87% similar at the amino acid level within the 4 protein interaction domains (Fig. S9). Treatment with either HR-CTGF-FL or HR-CTGF-CT enhanced zebrafish spinal cord regeneration (Fig. 3, G-I and Fig. S8C). Swim capacity was improved in HR-CTGF-FL- or -CT-treated animals by 2 wpi (Fig. 3G), near that of uninjured animals. As early as 1 wpi, we observed increased GFAP expression and more than 10-fold enhanced glial bridging in HRCTGF-treated animals compared to vehicle-treated controls (Fig. S8D). Bridging remained ~3-fold greater at 2 wpi (Fig. 3H). At 4 wpi, axon regeneration was increased by ~2-to-3-fold in HR-CTGF-FL- or -CT- treated animals compared to vehicle controls (Fig. 3I). Application of exogenous CTGF protein at the lesion site rescued functional and anatomical spinal cord regeneration in ctgfa mutants, indicating specificity of both the phenotype and treatment (Fig. S10). Thus, human CTGF protein, ostensibly via its C-terminal domains, enhances spinal cord regeneration in zebrafish.
Glial cell responses are thought to dictate the outcomes of spinal cord injury across species. We found that Ctgfa promotes zebrafish spinal cord regeneration, at least in part by facilitating the proliferation and bridging activity of pioneer glial cells that also express ctgfa. Other cell types induce ctgfa as well in the injured zebrafish spinal cord and might contribute additional functions during regeneration. In mammals, reactive gliosis causes scarring and inhibits regeneration (13, 14), although evidence indicates that the lesion contains a heterogeneous pool of glial cells that release both axon growth-inhibiting and -permissive factors (6, 15-19). We suggest that identifying the mammalian glial subtype that can produce CTGF or is competent to respond to it could reveal a pro-regenerative mammalian counterpart to the zebrafish bridging glia.
Supplementary Material
One Sentence Summary.
A connective tissue growth factor serves the unusual regenerative capacity of the zebrafish central nervous system.
Acknowledgments
We thank A. Johnson, C. Eroglu and M. Bagnat for discussions; N. Lee and K. Jones for technical and bioinformatics help; and the Duke University School of Medicine Zebrafish Shared Resource for animal care.
M.H.M. was supported in part by the NIH training grant T32HL007101. This research was supported in part by a grant from NIH (R01 HL081674 to K.D.P.) and by funds from the Max Planck Society (C.P. and D.Y.R.S.) and Duke University School of Medicine (K.D.P.).
RNA sequencing data are archived at GEO (Accession number GSE77025).
M.H.M. and K.D.P. are inventors on a pending patent on the use of Ctgf to enhance regeneration.
Footnotes
Supplementary Materials:
Materials and Methods
Figures S1-S10
Table S1
References (20-26)
References and Notes
- 1.Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol. 1997;377:577–595. doi: 10.1002/(sici)1096-9861(19970127)377:4<577::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Hui SP, Dutta A, Ghosh S. Cellular response after crush injury in adult zebrafish spinal cord. Dev Dyn. 2010;239:2962–2979. doi: 10.1002/dvdy.22438. [DOI] [PubMed] [Google Scholar]
- 3.Goldshmit Y, et al. Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J Neurosci. 2012;32:7477–7492. doi: 10.1523/JNEUROSCI.0758-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reimer MM, et al. Motor neuron regeneration in adult zebrafish. J Neurosci. 2008;28:8510–8516. doi: 10.1523/JNEUROSCI.1189-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin CY, Lee YS, Lin VW, Silver J. Fibronectin inhibits chronic pain development after spinal cord injury. J Neurotrauma. 2012;29:589–599. doi: 10.1089/neu.2011.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tom VJ, Doller CM, Malouf AT, Silver J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J Neurosci. 2004;24:9282–9290. doi: 10.1523/JNEUROSCI.2120-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertel M, Tretter Y, Alzheimer C, Werner S. Connective tissue growth factor: a novel player in tissue reorganization after brain injury? Eur J Neurosci. 2000;12:376–380. doi: 10.1046/j.1460-9568.2000.00930.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, et al. Accumulation of connective tissue growth factor+ cells during the early phase of rat traumatic brain injury. Diagn Pathol. 2014;9:141. doi: 10.1186/1746-1596-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad S, Schluesener HJ, Adibzahdeh M, Schwab JM. Spinal cord injury induction of lesional expression of profibrotic and angiogenic connective tissue growth factor confined to reactive astrocytes, invading fibroblasts and endothelial cells. J Neurosurg Spine. 2005;2:319–326. doi: 10.3171/spi.2005.2.3.0319. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development. 2011;138:3421–3430. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 12.Robinson PM, et al. Proteolytic processing of connective tissue growth factor in normal ocular tissues and during corneal wound healing. Invest Ophthalmol Vis Sci. 2012;53:8093–8103. doi: 10.1167/iovs.12-10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raposo C, Schwartz M. Glial scar and immune cell involvement in tissue remodeling and repair following acute CNS injuries. Glia. 2014;62:1895–1904. doi: 10.1002/glia.22676. [DOI] [PubMed] [Google Scholar]
- 15.Waselle L, Quaglia X, Zurn AD. Differential proteoglycan expression in two spinal cord regions after dorsal root injury. Mol Cell Neurosci. 2009;42:315–327. doi: 10.1016/j.mcn.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 16.White BD, et al. Beta-catenin signaling increases in proliferating NG2+ progenitors and astrocytes during post-traumatic gliogenesis in the adult brain. Stem Cells. 2010;28:297–307. doi: 10.1002/stem.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson MA, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meletis K, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill SJ, Barbarese E, McIntosh TK. Regional heterogeneity in the response of astrocytes following traumatic brain injury in the adult rat. J Neuropathol Exp Neurol. 1996;55:1221–1229. doi: 10.1097/00005072-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 21.Cermak T, Starker CG, Voytas DF. Efficient design and assembly of custom TALENs using the Golden Gate platform. Methods Mol Biol. 2015;1239:133–159. doi: 10.1007/978-1-4939-1862-1_7. [DOI] [PubMed] [Google Scholar]
- 22.Trapnell C, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennis G, Jr., et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 24.Wang J, Karra R, Dickson AL, Poss KD. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev Biol. 2013;382:427–435. doi: 10.1016/j.ydbio.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poss KD, Nechiporuk A, Hillam AM, Johnson SL, Keating MT. Mps1 defines a proximal blastemal proliferative compartment essential for zebrafish fin regeneration. Development. 2002;129:5141–5149. doi: 10.1242/dev.129.22.5141. [DOI] [PubMed] [Google Scholar]
- 26.Yu Y, Schachner M. Syntenin-a promotes spinal cord regeneration following injury in adult zebrafish. Eur J Neurosci. 2013;38:2280–2289. doi: 10.1111/ejn.12222. Each reference should be on a separate line ending in a period. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.