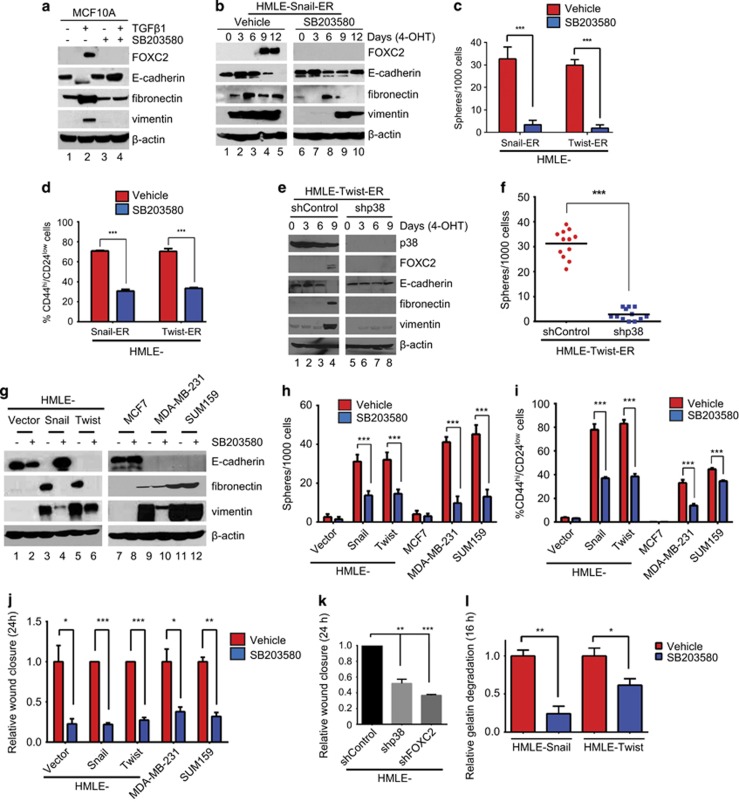
Figure 3.
p38 inhibition compromises the acquisition and maintenance of EMT and stem cell properties in vitro. (a) MCF10A cells were treated with TGFβ1 alone, or in combination with SB203580, for 3 days. The cells were harvested, and the corresponding lysates were analyzed by immunoblotting for FOXC2, E-cadherin and mesenchymal markers. β-Actin was used as a loading control. (b) HMLE-Snail-ER cells were treated with 4-OHT for 12 days and concurrently exposed to vehicle or SB203580. Cells were harvested at the indicated time points and the corresponding lysates were analyzed by immunoblotting for FOXC2, E-cadherin and mesenchymal markers. β-Actin was used as a loading control. (c) HMLE-Snail-ER and HMLE-Twist-ER cells were treated with 4-OHT for 12 days and concurrently exposed to vehicle or SB203580. One thousand cells were seeded per well in ultra-low attachment plates and cultured for 7–10 days. Spheres with a diameter >75 μm were counted. The data are reported as the number of spheres formed/1000 seeded cells±s.e.m. (d) The percentage of CD44high/CD24low cells in 4-OHT-treated HMLE-Snail-ER and HMLE-Twist-ER populations, concurrently exposed to vehicle or SB203580, was determined by fluorescence-activated cell sorting (FACS). Data are presented as mean±s.e.m. (e) HMLE-Twist-ER cells, transduced with p38 shRNA (shp38) or control shRNA (shControl), were treated with 4-OHT for 9 days and harvested at the indicated time points. The corresponding lysates were analyzed by immunoblotting for FOXC2, E-cadherin and mesenchymal markers. β-Actin was used as a loading control. (f) The sphere-forming efficiency of 4-OHT-treated HMLE-Twist-ER cells, transduced with control shRNA (shControl) or p38 shRNA (shp38), was determined. The data are reported as the number of spheres formed/1000 seeded cells±s.e.m. (g) The indicated cells were treated with vehicle or SB203580, and the corresponding lysates were analyzed by immunoblotting for FOXC2, E-cadherin and mesenchymal markers. β-Actin was used as a loading control. (h) The sphere-forming efficiency of the indicated cells was determined in the presence of vehicle or SB203580. The data are reported as the number of spheres formed/1000 seeded cells±s.e.m. (i) The percentage of CD44high/CD24low subpopulations in the indicated cells, treated with vehicle or SB203580, was determined by FACS. Data are presented as mean±s.e.m. (j) The relative wound closure by the indicated cells, treated with vehicle or SB203580, was measured by image analysis and represented in a graphical format. Data are presented as mean±s.e.m. (k) The relative wound closure by HMLE cells, transduced with control shRNA (shControl), p38 shRNA (shp38) or FOXC2 shRNA (shFOXC2), was measured by image analysis and represented in a graphical format. Data are presented as mean±s.e.m. (l) HMLE-Snail and HMLE-Twist cells were plated on fluorescein isothiocyanate (FITC)-conjugated gelatin and treated with vehicle or SB203580. After 16 h, the cells were fixed and stained with fluorescent phalloidin, and the nuclei were counterstained with DAPI to facilitate visualization of the cells (see Supplementary Figure S4b). Degradation of FITC–gelatin was quantified by image analysis. n=150 cells/sample. Data are reported as mean±s.e.m. P-values were calculated using Student's unpaired two-tailed t-test. *P<0.05; **P<0.01; ***P<0.001 compared with the control.