Figure 7.
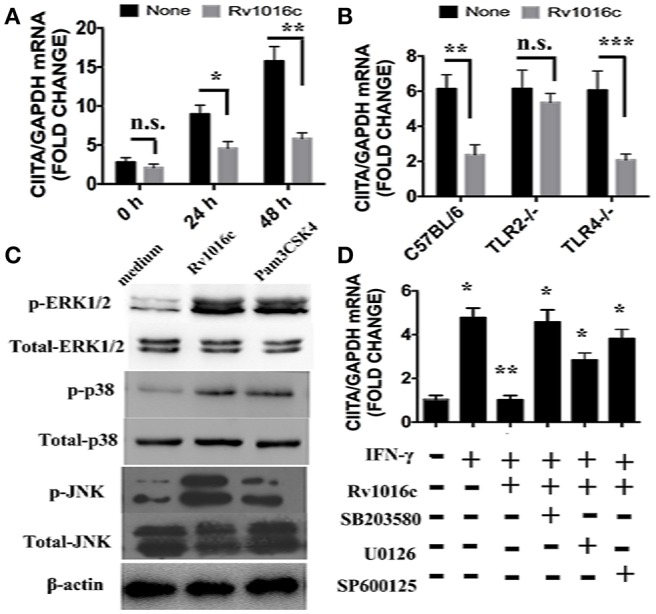
Rv1016c inhibits IFN-γ- induced expression of CIITA dependent on TLR2 and MAPK pathway. (A) THP-1 cells were incubated with or without Rv1016c-His (20 μg/ml) for 24 h, then with IFN-γ (15 ng/ml) in the continued presence or absence of Rv1016c for the indicated times. RNA was isolated, and quantitative real-time PCR was used to analyze the expression of CIITA. (B) Macrophages (1 × 106cells/well) from wild type, TRL2−/− or TRL4−/− were incubated with or without Rv1016c-His (20 μg/ml) for 24 h, then with IFN-γ (15 ng/ml) in the continued presence or absence of Rv1016c-His for the indicated times. The expression of CIITA was measured as previously described. (C) Rv1016c activates MAPKs pathway in macrophages. THP-1 cells were treated with Rv1016c (20 μg/ml) or Pam3CSK4 (5 μg/ml) for 1 h, the phosphorylation of p38, ERK (1/2) and JNK were examined by blotting with specific antibodies to p-p38, p38, p-ERK1/2, ERK1/2, p-JNK, and JNK. (D) Rv1016c-mediated inhibition of CIITA expression is dependent on MAPK signaling. Macrophages were incubated with or without DMSO (vehicle control), p38 (SB203580, 10 μM), ERK (U0126, 10 μM), or JNK (SP600125, 10 μM) for 1 h at 37°C. Rv1016c (20 μg/ml) was added to samples for the next 6 h, and IFN-γ (15 ng/ml) was added for a final 5 h (presence of inhibitors maintained). Expression of CIITA mRNA was assayed as previously described. All samples were first normalized to GAPDH and are expressed as the fold change compared with untreated controls. All data are expressed as the mean ± SD from three separate experiments. (*p < 0.05, **p < 0.01, ***p < 0.001 vs. corresponding controls).
