FIGURE 3.
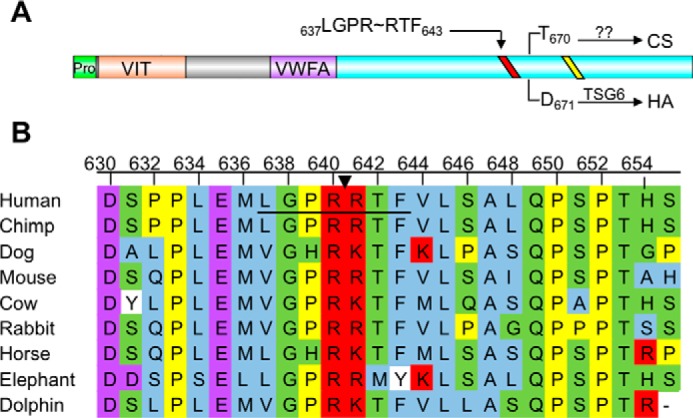
HC1 contains a conserved thrombin cleavage sequence proximal to the glycosaminoglycan attachment site. A, domain structure of inter-α-inhibitor HC1. HC1 has two known domains, the vault protein inter-α-trypsin (VIT) domain and a von Willebrand type A (VWFA) domain. The site of CS linkage or HA attachment (for TSG-6-mediated transfer) is depicted by an arrow from the indicated residue to either CS or HA. The conserved sequence required for processing by an unknown Golgi enzyme and covalent transfer to CS is depicted as a yellow line. The predicted thrombin consensus sequence (LGPRRTF) is shown as a red line. B, partial HC1 sequence alignment of the thrombin consensus sequence. The consensus sequence is underlined, and an arrow indicates the scissile bond. Amino acids are colored corresponding to the following properties. Blue, small and hydrophobic residues; purple, acidic residues; red, basic residues; green, hydroxyl, sulfhydryl, amine, and glycine; yellow, proline; white, aromatic.
