FIGURE 2.
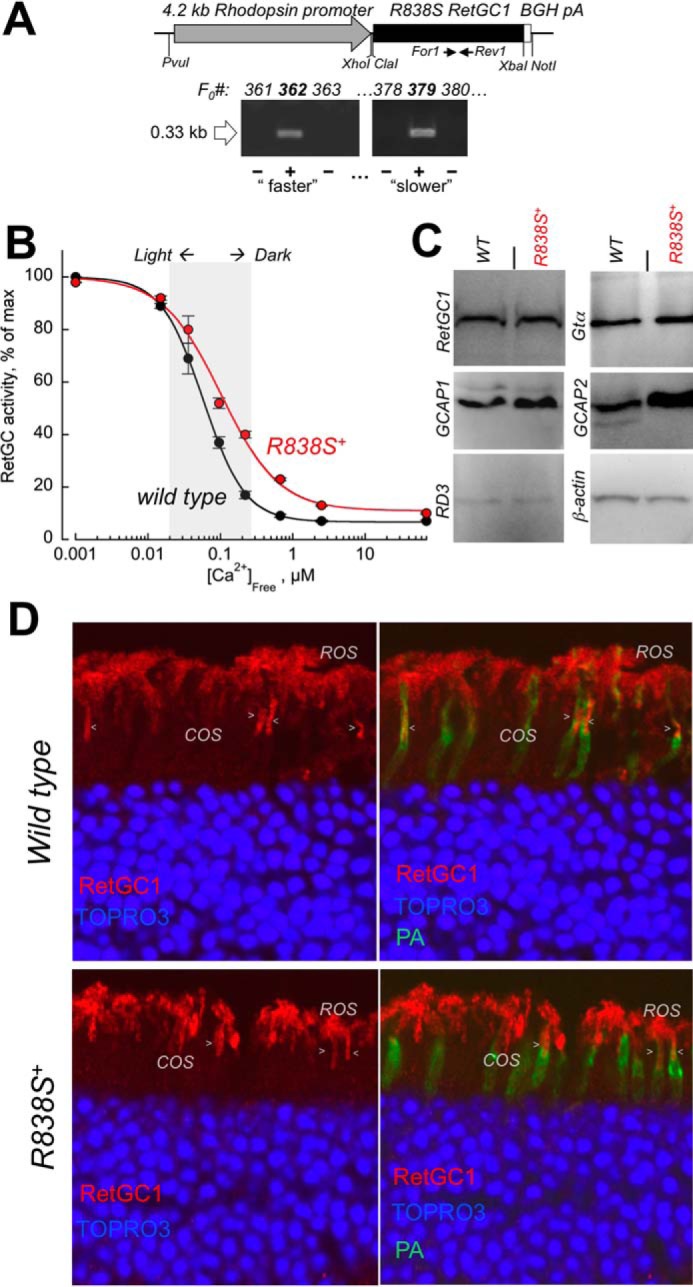
Transgenic expression of human GUCY2D-CORD6 R838S RetGC1 in vivo alters calcium sensitivity of cGMP synthesis in a mouse retina. A, schematics of the R838S RetGC1 transgene expressing construct. The 3.6-kbp R838S RetGC1 cDNA was inserted between the 4.2-kbp mouse rod opsin gene fragment harboring the promoter region and the 0.3-kb BGH polyadenylation signal-containing fragment as described under “Experimental Procedures.” The presence of the transgenic construct in the tail DNA samples from F0 mice was tested by PCR amplification of the transgene-specific 0.33-kb product, between the positions of the For1 and Rev1 primers as indicated in the upper panel and described under “Experimental Procedures.” Lines developing progressive blindness (slower in line 379 and faster in line 362) were derived from the two positive founders and analyzed as described further under “Results.” B, Ca2+ sensitivity of the cGMP synthesis rate in the dark-adapted retinas extracted from 3-week-old transgene-positive (R838S+, red symbols) and transgene-negative (wild type, black symbols) littermate retinas of the slower R838S RetGC1 line 379, assayed at the indicated free Ca2+ concentrations as described under “Experimental Procedures.” The data (mean ± S.D.) were normalized as the percent of maximal catalytic activity of the cyclase in each preparation and fitted with the Hill function, A = (Amax − Amin)/(1 + ([Ca]/K½Ca)H) + Amin, where Amax and Amin are the respective maximal and minimal activity, [Ca], free Ca2+ concentration; K½Ca, free Ca2+ concentration causing 2-fold inhibition of the cyclase activity; and H, the Hill coefficient. The average K½Ca in five independently tested transgenic mice (R838S+) increased to 110 ± 26 from 59 ± 14 nm in four wild type siblings (p < 0.03). The shaded area approximates the physiological range of the free Ca2+ concentrations in photoreceptors between light-adapted and dark-adapted states (73). C, Western immunoblotting of the retinas from 3-week-old wild type (WT) and transgene-positive (R838S+) siblings probed for RetGC1, GCAP1, GCAP2, RD3, Gtα, and β-actin. D, transgenic expression of the CORD6 RetGC1 mutant does not change the overall pattern of RetGC1 immunofluorescence in the R838S+ retina. Paraformaldehyde-fixed retina sections from 3-week-old littermates, either wild type (upper panels) or transgene-positive (bottom panels), were probed with anti-RetGC1 antibody (red) and peanut agglutinin (PA, green); the nuclei were counterstained with TOPRO3 iodide (pseudo-blue). The arrowheads indicate anti-RetGC1 fluorescence in cone outer segments (COS), below the rod outer segment (ROS) layer, identified by the surrounding PA-stained cone sheaths superimposed on the anti-RetGC1 fluorescence (right panels). The samples were processed and stained in parallel and the images were recorded in the same experiment, using identical settings for the anti-RetGC1 fluorescence imaging.
