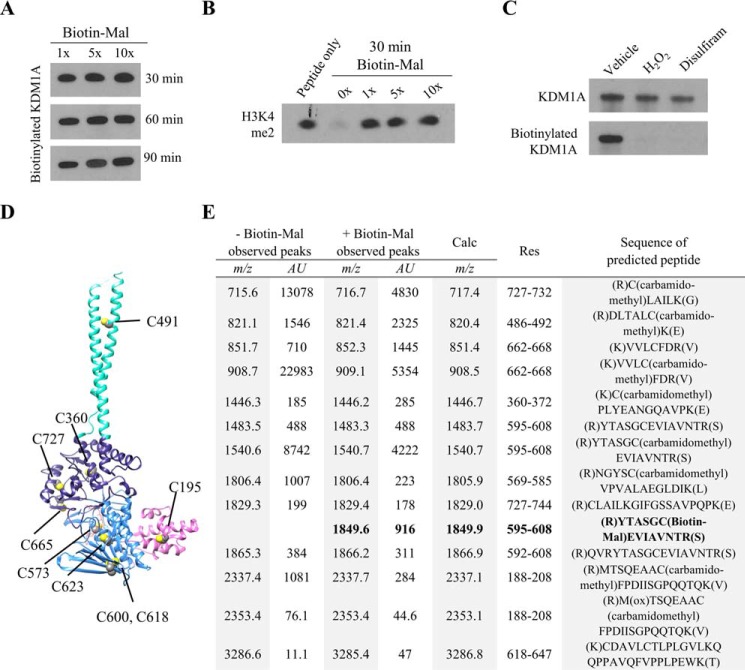
FIGURE 4.
MALDI-TOF analysis of KDM1A tryptic digests identifies Cys-600 as a site of Biotin-Mal labeling. A, recombinant KDM1A (5 μm) was pre-reduced, desalted with buffer exchange, and labeled with various equivalents of Biotin-Mal for 30, 60, or 90 min. The extent of thiol labeling was monitored by blotting with a streptavidin-HRP conjugate. B, inhibition of recombinant KDM1A by Biotin-Mal labeling was determined by Western blot analysis of the depletion of H3K4me2 starting material. Recombinant KDM1A was pre-incubated with Biotin-Mal for 30 min prior to demethylation of H3K4me2 peptide substrate for 1 h. C, pre-treatment of recombinant KDM1A with H2O2 or disulfiram followed by desalting with buffer exchange blocks labeling with Biotin-Mal, as measured by blotting with a streptavidin-HRP conjugate. D, crystal structure (PDB ID: 2HKO) indicating the locations of the nine cysteine residues of KDM1A. Cys-195 is in the SWIRM domain (pink), Cys-573, -600, -618, and -623 are in the FAD-binding amine oxidase domain (light blue), Cys-360, -665, and -727 are in the substrate-binding amine oxidase domain (dark blue), and Cys-491 is in the tower domain (teal). E, tabular summary of cysteine-containing tryptic KDM1A peptides identified by MALDI-TOF. Recombinant KDM1A (5 μm) was pre-reduced and desalted with buffer exchange, then labeled with 10 μm Biotin-Mal for 20 min prior to in-gel alkylation and digestion.