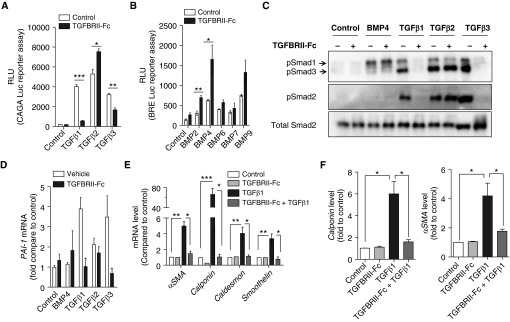
Figure 1.
TGFBRII-Fc selectively inhibits transforming growth factor-β1 (TGF-β1)- and TGF-β3–induced signaling and blocks TGF-β–induced phenotypic modulation of smooth muscle cells. (A) HEK293 cells stably expressing a TGF-β–responsive CAGA-luciferase (Luc) reporter transgene and (B) C2C12 cells stably expressing a bone morphogenetic protein (BMP) response element reporter (BRE)–Luc reporter transgene were incubated in the presence or absence of TGFBRII-Fc (2 μg/ml) for 30 minutes before stimulation with various BMP ligands (10 ng/ml) or TGF-β1, -2, or -3 (1 ng/ml) overnight, revealing selective inhibition by TGFBRII-Fc of TGF-β1– and TGF-β3– but not TGF-β2–induced activation of CAGA-luciferase activity, and slightly increased BRE-luciferase activity in response to BMP2 and BMP4 in the presence of TGFBRII-Fc. Human pulmonary artery smooth muscle cells were deprived of serum overnight, pretreated with TGFBRII-Fc (2 μg/ml), followed by incubation with BMP4 (10 ng/ml), TGF-β1, -2, or -3 (1 ng/ml of each for 30 min), and analyzed by immunoblot for (C) phosphorylated Smads (pSmad) 1, 2, and 3 and (D) for mRNA expression of the TGF-β transcriptional target PAI-1 by quantitative reverse transcriptase polymerase chain reaction. TGFBRII-Fc inhibited SMAD2 and SMAD3 activation, CAGA-Luc reporter activity, and Pai-1 mRNA expression via TGF-β1 and TGF-β3, but not TGF-β2. After stimulation with TGF-β1, the relative levels of (E) mRNA and (F) protein expression of smooth muscle cell contractile markers were examined at 24 and 72 hours, respectively. Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 as indicated. αSMA = α-smooth muscle actin; PAI-1 = plasminogen activator inhibitor 1; RLU = relative light units.