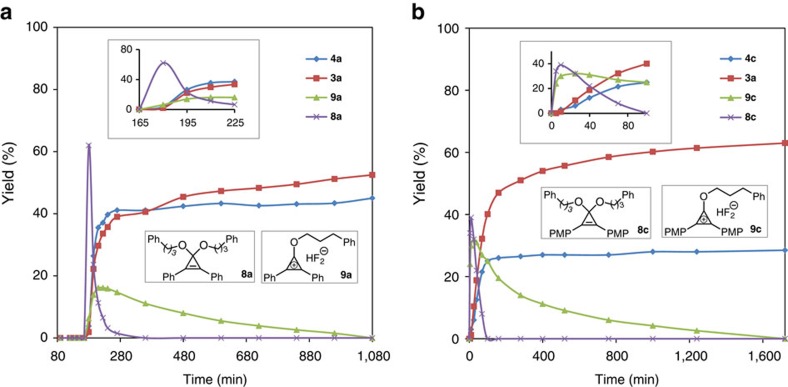
Figure 5. Mechanistic study on the deoxyfluorination of alcohols with CpFluors.
CpFluors with electron-netural (1a) and electron-rich (1c) aryl substitutents were chosen to monitor the influence of electronic nature on the chemical outcome. Both reactions were perforformed in PTFE NMR tubes with DCE-d4 as the solvent. (a,b) Yield profiles of products and intermediates generated in the reactions of 3-phenylpropan-1-ol (2a, 1.0 equiv.) (a,b) with CpFluors 1a (1.0 equiv.) (a) and 1c (1.0 equiv.) (b) during the whole monitoring process. Insets on the top (a,b): the enlarged yield profiles at the early stage of the reactions. PMP, p-methoxyphenyl.