Abstract
Childhood apraxia of speech (CAS) is a paediatric speech sound disorder in which precision and consistency of speech movements are impaired. Most children with idiopathic CAS have normal structural brain MRI. We hypothesize that children with CAS have altered structural connectivity in speech/language networks compared to controls and that these altered connections are related to functional speech/language measures.
Whole brain probabilistic tractography, using constrained spherical deconvolution, was performed for connectome generation in 17 children with CAS and 10 age-matched controls. Fractional anisotropy (FA) was used as a measure of connectivity and the connections with altered FA between CAS and controls were identified. Further, the relationship between altered FA and speech/language scores was determined.
Three intra-hemispheric/interhemispheric subnetworks showed reduction of FA in CAS compared to controls, including left inferior (opercular part) and superior (dorsolateral, medial and orbital part) frontal gyrus, left superior and middle temporal gyrus and left post-central gyrus (subnetwork 1); right supplementary motor area, left middle and inferior (orbital part) frontal gyrus, left precuneus and cuneus, right superior occipital gyrus and right cerebellum (subnetwork 2); right angular gyrus, right superior temporal gyrus and right inferior occipital gyrus (subnetwork 3). Reduced FA of some connections correlated with diadochokinesis, oromotor skills, expressive grammar and poor lexical production in CAS.
These findings provide evidence of structural connectivity anomalies in children with CAS across specific brain regions involved in speech/language function. We propose altered connectivity as a possible epiphenomenon of complex pathogenic mechanisms in CAS which need further investigation.
Keywords: Childhood apraxia of speech, Connectivity, Diffusion magnetic resonance, Fractional anisotropy, Diadochokinesis
Highlights
-
•
Connectivity anomalies are present in children with Childhood Apraxia of Speech.
-
•
Connectivity anomalies include brain regions involved in speech/language function.
-
•
Altered connectivity correlates with a measure of speech/language dysfunction.
1. Introduction
Childhood apraxia of speech (CAS) is a severe and persistent paediatric motor speech sound disorder in which, according to the American Speech and Hearing Association (ASHA 2007), planning and programming of movements that underly speech are impaired in the absence of neuromuscular deficits (e.g. abnormal reflexes, abnormal tone). Children with CAS display reduced speech timing and sequencing skills and show particular difficulties in dynamic transitions between articulatory postures and in combining smaller units of movement into larger ones. CAS can be secondary to neurological diseases (i.e. epilepsy, brain lesion, metabolic deficits) or can present as an idiopathic disorder. To date, the aetiology and underlying neural mechanisms of idiopathic CAS remain largely unknown (Liégeois and Morgan, 2012, Liégeois et al., 2014).
Functional MRI studies in healthy adults suggest that different brain regions contribute to speech planning (Riecker et al., 2005): the medial frontal cortex, anterior insula, dorsolateral frontal cortex and superior cerebellum. Consistent with this hypothesis, apraxia of speech in adults was found to be strongly related to frank lesions of the left hemisphere, mainly due to brain infarcts (Liégeois and Morgan, 2012), involving the posterior part of Broca's region (Hillis et al., 2004, Jordan and Hillis, 2006), the frontal insular cortex (Dronkers, 1996, Nagao et al., 1999) or its adjacent white matter (Ackermann and Riecker, 2010, Ogar et al., 2005). Much less is known about paediatric motor speech planning disorders. In contrast to what is reported in adults, the majority of children with idiopathic or symptomatic (e.g. related to epilepsy or metabolic disorders) CAS were found to have normal structural brain MRI on conventional imaging, suggesting that brain abnormalities that underly idiopathic CAS might be too subtle to be detected by clinical MRI (Liégeois and Morgan, 2012). Indeed, emerging evidence from studies using more advanced quantitative measures of structural MRI in speech disorders support the presence of structural brain abnormalities on a microscopic level. Using voxel-based morphometry, morphological abnormalities were found in supramarginal gyrus and bilaterally in the planum temporale and in Heschl's gyrus for children with a subtype of speech sound disorder characterized by persistent speech sound errors (Preston et al., 2014). In children with idiopathic CAS, a thicker left supramarginal gyrus was found compared to controls by Kadis et al. (2014), in the absence of appreciable macroscopic lesions from a neuroanatomical qualitative approach (Kadis et al., 2014).
Overall, these findings have been interpreted as the possible result of an immature or altered development of connectivity, where a thicker cortex may reflect the lack of physiological synaptic pruning that normally occurs after the first year of life (Alexander-Bloch et al., 2013, Lerch et al., 2006, Preston et al., 2014). However, no direct evidence supporting these hypotheses is available, as no study has directly explored white matter connectivity in CAS. Diffusion MRI provides a non-invasive tool to explore white matter microstructure. It allows the delineation of white matter pathways using tractography, and subsequent assessment of microstructure within three-dimensional tracts. More recently, methods have been developed to assess whole brain structural connectivity more comprehensively, the connectome analysis, with no a priori hypotheses regarding tracts of interest (Hagmann et al., 2005, Pannek et al., 2014).
In this study, our aim was to investigate structural connectivity in children with CAS using connectome analysis. To achieve this, we performed whole brain probabilistic tractography based on an advanced diffusion imaging analysis with constrained spherical deconvolution. We compared fractional anisotropy (FA) as a measure of altered white matter connectivity in children with CAS and age-matched controls. We hypothesized that children with CAS had lower FA in a number of connections involved in speech/language function. Further, we investigated the relationship between altered FA and clinical measures of speech/language function. We hypothesized that connections with altered FA were related to functional speech/language impairment.
2. Materials and methods
2.1. Participants
Children with idiopathic CAS were selected from a large population of subjects (n = 70) consecutively referred to the Neurolinguistic laboratory of the IRCCS Stella Maris for speech/language disorders between January 2013 and October 2015. CAS diagnosis was conducted by a multidisciplinary team on the basis of a comprehensive clinical, instrumental and neurological assessment and video recorded speech–language evaluation. To consistently specify the characteristics of our sample according to international CAS criteria, video recorded speech samples were analysed by two independent expert observers (AC, PC) according to a checklist including ASHA criteria (ASHA 2007) and Strand's features of CAS (Murray et al., 2015, Shriberg et al., 2011) (see Table 1). The agreement of the two raters (AC, PC) was excellent both for Kappa statistic (Kappa = 0.83, 95% CI = 0.62–1.00) and for Intra-class Correlation Coefficient (ICC = 0.83, 95% CI = 0.60–0.94 ) (Landis and Koch, 1977).
Table 1.
Presence/absence of ASHA and Strand features for assigning diagnosis of childhood apraxia of speech. Following the Murray et al. (2015) procedure, the first and second Strand features were incorporated into the second ASHA (2007) criterion. The third Strand feature was incorporated into the third ASHA criterion.
| Subject | Age (years) | Gender | Handedness | Inconsistent errors on consonants and vowels (ASHA 1st criterion) | Lengthened and disrupted co-articulatory transitions between sounds and syllables (ASHA 2nd criterion/Strand 1st and 2nd criteria) |
Inappropriate prosody (ASHA 3rd criterion Strand 3rd criterion) |
Vowel or consonant distortions including distorted substitutions (Strand 4th criterion) |
Groping (Strand 5th criterion) |
Intrusive schwa (Strand 6th criterion) |
Voicing errors (Strand, 7th criterion) |
Slow rate (Strand, 8th criterion) | Slow DDK rate (Strand 9th criterion) | Increased difficulty with longer or phonetically more complex words (Strand 10th criterion) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.1 | M | R | + | + | + | + | + | − | + | + | + | + |
| 2 | 7.0 | M | R | + | + | + | + | + | − | + | + | + | + |
| 3 | 6.2 | M | R | + | + | + | + | + | − | + | + | + | + |
| 4 | 6.2 | F | R | + | − | + | + | + | − | − | − | + | + |
| 5 | 6.0 | M | L | + | + | + | + | + | − | + | + | + | + |
| 6 | 6.0 | F | R | + | + | − | + | − | − | + | + | + | + |
| 7 | 9.6 | M | R | + | + | + | + | + | + | + | + | + | + |
| 8 | 5.5 | F | R | + | + | + | + | + | − | + | + | + | + |
| 9 | 7.0 | M | R | + | + | + | + | + | − | + | + | + | + |
| 10 | 4.9 | M | R | + | + | + | + | − | − | + | − | + | + |
| 11 | 17 | M | R | + | + | + | − | + | − | + | + | + | + |
| 12 | 6.4 | M | R | + | + | + | + | + | − | + | + | + | + |
| 13 | 5.2 | F | R | + | + | − | + | − | − | − | + | + | + |
| 14 | 6.6 | M | R | + | + | − | + | + | − | + | + | + | + |
| 15 | 5.1 | M | R | + | + | + | + | − | − | − | − | + | + |
| 16 | 6.7 | M | R | + | + | + | + | + | − | + | + | + | + |
| 17 | 6.6 | M | L | + | + | + | + | + | − | − | + | + | + |
F: female; M: male; R: right; L: left.
Exclusion criteria were the presence of orofacial structural abnormalities, known pathologies of neurological, neurometabolical and genetic etiologies, audiological deficits and epilepsy. All subjects were screened for standard karyotype and Fraxa-e mutations. None of the CAS participants presented with any obvious signs of neurological deficit, such as hypotonia or hypertonia, jaw-jerk reflex or hyporeflexia, dyskinetic movements, abnormal vegetative functions, paresis, loudness problems or pathological weakness of orofacial and velopharyngeal muscles. Nonverbal orofacial movements were possible, but not accurate in about half of the sample qualitatively deemed incorrect. Moreover, single and automatic movements were better preserved than sequential and voluntary ones. At structural brain MRI, none of the CAS participants showed brain lesions as reported by an experienced paediatric neuroradiologist (RP).
The final sample included seventeen children (13 males and 4 females, mean age 7.1 years, SD 2.8) with idiopathic CAS (see Table 1 for detailed single subject features supporting the CAS diagnosis). Fifteen of the selected participants were part of a group of children with CAS, recently described according to their clinical, genetic and structural MRI characteristics (Chilosi et al., 2015).
Ten healthy children (8 males and 2 females, mean age 7.1 years, age range 4–16, SD 2.3) were enrolled from the community as controls for the MRI protocol. They did not receive a formal speech language assessment, however none of them presented any abnormalities on neurological examination and at informal evaluation of speech and language abilities performed by a child neurologist (SF).
Parental consent and child assent were obtained in all cases. The study was approved by the Ethics Committee of the IRCCS Fondazione Stella Maris (Number 13/2013).
2.2. MRI
MRI data were acquired using a 1.5T MR scanner (GE, Signa Horizon 1.5, Milwaukee, WI, USA). High-resolution structural images were acquired using a 0.9 mm isotropic 3D T1 BRAVO sequence with TR/TE 12.36/5.18 ms; flip angle 13; for an acquisition time of 4.5 min. Diffusion images were acquired using a commercial single shot echo planar multi-direction diffusion weighted sequence, employing a dual bipolar diffusion gradient and a double spin echo. Imaging parameters were: 45 axial slices; 3.0 × 3.0 mm in-plane resolution; 3 mm slice thickness; field of view = 24 × 24 cm; TR/TE = 10,000/92 ms; acquisition matrix = 80 × 80; 30 encoding gradients distributed in space using the electrostatic approach with a b-value of 1000 s/mm2 and with one image at minimal diffusion weighting (b = 0). The acquisition time for diffusion data was 7 min.
2.3. Structural parcellation and connectome generation
Volumetric segmentation was based on an atlas registration. The T1-weighted images of an MNI Anatomical Atlas Labelling (AAL) atlas (Tzourio-Mazoyer et al., 2002) with 116 cortical/subcortical parcellated regions was non-rigidly registered to each the subject's 3D T1 BRAVO volume (Modat et al., 2010). Registration was carefully assessed visually for each subject. The subject's 3D T1 volume was then rigidly registered to the subject's FA map obtained through diffusion imaging (Ourselin et al., 2002). The combined transformation was then applied to MNI AAL cortical parcellations to propagate labels into the single subject's diffusion space. The AAL parcellation contained a total of 116 cortical, sub-cortical, cerebellar and thalamic structures. In order to prevent diffusion tractography streamlines from crossing the cortical folds, a termination mask was generated. Diffusion images were preprocessed as described previously (Pannek et al., 2014). Motion artefacts and within-volume movement were detected and corrected. Signal intensity outlier voxels (caused by cardiac pulsation, bulk head motion and other artefacts) were detected and replaced and between-volume registration for head movement was performed (Pannek et al., 2014). After preprocessing, FA was estimated from the corrected diffusion data. Constrained spherical deconvolution was used to estimate fibre orientation distribution for tractography (Tournier et al., 2008). Five million probabilistic streamlines were generated to create a whole brain tractogram. Intrahemispheric and interhemispheric connections were extracted from the whole brain tractogram by hit-testing both terminal endpoints of every streamline with every cortical, subcortical and cerebellar region. Mean FA values were calculated by sampling the FA maps at every voxel of selected streamlines and encoded in a 116 × 116 connectivity matrix.
2.4. Speech and language assessment
Speech/language assessment was based on a comprehensive battery of tests including parental report on medical case history, early vocal behaviour and speech-language developmental milestones, evaluation of isolated and sequential verbal and nonverbal oromotor skills, accuracy and consistency of phonological errors, diadochokinesis, expressive grammar and receptive/expressive vocabulary.
A detailed description of the tests included in this battery is reported in Table 2.
Table 2.
Task description for individual measures of speech/language clinical assessment in children with CAS.
| Task | Description |
|---|---|
| Parental report on case history | Family history, child's pre-, peri- and post-natal clinically significant events, early vocal behaviour, language milestones acquisition (modified version of the questionnaire reported by Chilosi et al., 2009). |
| Phonetic inventory | Repetition of 21 syllables containing all the Italian consonantal sounds |
| Oromotor skills | Assessment of isolated and sequenced volitional verbal and nonverbal oral movements (Bearzotti and Fabbro, 2003) |
| Accuracy | Picture naming test (Bello et al., 2010) and repetition of six three-syllable non-words (/tapaka/, /pataka/, /takapa/, /kapata/, /pakata/ and /katapa/). Scoring based on percentage of erroneous productions in both tasks (only errors of pronunciation) |
| Consistency | Picture naming test (Bello et al., 2010) and repetition of six three-syllable non-words (/tapaka/, /pataka/, /takapa/, /kapata/, /pakata/ and /katapa/). Scoring based on the percentage of variable phonetic errors in two repeated productions of the same word or non-word stimuli. |
| Diadochokinesis | Maximum performance rate: fast repetition for 20 s of the trisyllabic non-word sequence /pataka/ |
| Expressive grammar | Analysis of the level of grammatical organization of spontaneous speech according to a six-level rating system, based on stages of grammar acquisition in Italian-speaking children (Cipriani et al., 1993, Chilosi et al., 2013) |
| Receptive vocabulary | Phono Lexical Test-TFL (Vicari, 2007), PPVT-III (Dunn and Dunn, 1997), depending on the child's age and on the severity of the disorder |
| Expressive vocabulary | Phono Lexical Test-TFL (Vicari, 2007) and/or One-Word Picture Vocabulary Test (Brizzolara, 1989), depending on the child's age and on the severity of the disorder |
2.5. Statistical analysis
Statistical analysis of the brain network was performed using the Network-Based Statistics (NBS; Zalesky et al., 2010) toolbox for Matlab (https://sites.google.com/site/bctnet/comparison/nbs). A general linear model was used to identify differences in FA between CAS and control groups for every connection, using age as a confounding variable. We hypothesized that FA would be reduced in children with CAS compared to healthy children, reflecting WM microstructure impairment in some regions involved in speech/language processing. For consistency, we tested the opposite hypothesis of a possible reduction of FA in some regions in controls compared to CAS.
A t-threshold of 4.3 was used for individual connections. Correction for multiple comparisons was performed using NBS (Zalesky et al., 2010). This approach identifies statistically significant clusters of connections (subnetworks), correcting for multiple comparison with a greater power than Bonferroni correction or false discovery rate (Zalesky et al., 2010). For each cluster, subnetworks were displayed in terms of nodes, as crucial interconnected regions with connections of altered FA. For subnetworks that showed significant alterations in FA in children with CAS compared to children with typical development, we performed an exploratory assessment of the relationship between the average FA value of the connections and clinical speech/language measures using a linear model (Bender and Lange, 2001). Statistical analyses were performed using R (R Development CoreTeam 2008).
3. Results
3.1. Case history and clinical characteristics of CAS
Based on parental reports, prenatal and perinatal history was normal in 12 subjects; some transient problems during pregnancy or delivery were reported in the remaining 5 subjects, but none suffered from moderate or severe foetal or neonatal complications. All children presented severe speech and language delay characterized by: a) quantitatively reduced and qualitatively abnormal babbling with delayed onset (mean age 14.2 months, SD 7.4 months) and sporadic production of a very restricted number of speech sounds; b) delayed emergence of first words (mean age 25.9 months, SD 11.4 months); c) very slow increase of vocabulary size with delay in acquisition of the first 50 words (mean age 49.8 months, SD 12.6) and late emergence of combinatory speech (mean age 52.3 months, SD 11.8); d) high percentage of unintelligible speech during preschool years (mean percentage of unintelligible utterances 65.6 ± 13.2%), as reported by parents.
Assessment of oromotor skills with nonverbal tasks revealed the presence of associated signs of nonverbal oromotor apraxia in more than half of the sample: 56% showed inaccurate execution of isolated nonverbal oromotor tasks (mean z-score − 3.5 ± 5.3), and 70% were also impaired in performing sequential nonverbal oromotor tasks (mean z-score − 3.2 ± 3.3). Phonetic inventory was markedly reduced (mean number of consonant phonemes on 21 tested 12.5 ± 4.0) with a high percentage of phonetically inaccurate productions of words (mean 71 ± 20%) and of inconsistent errors (mean 64 ± 24.4%). Diadochokinesis rate was significantly slower (p < 0.001) in children with CAS (mean score 10 ± 7.3) compared to normative data collected on 64 typically developing children with mean age of 6.4 yrs. (mean score 25.6 ± 3) (Chilosi et al., 2015).
Most children with CAS (95%) performed below age expectation in at least one language area. Expressive grammar was the most impaired domain with about 70% of children showing a primitive combinatorial or telegraphic language level, and only one child attaining an age-appropriate level of grammar organization. Lexical abilities were better preserved with about 56% and 47% of children performing in the normal-borderline range in receptive vocabulary (mean standard score 80.8 ± 15.4) and in expressive vocabulary (mean z-score − 1.3 ± 1.6), respectively.
3.2. Connectome comparison between CAS and controls
Significant differences in FA between the groups were found in three subnetworks, as shown in Fig. 1. In each case, FA was reduced in children with CAS compared to controls.
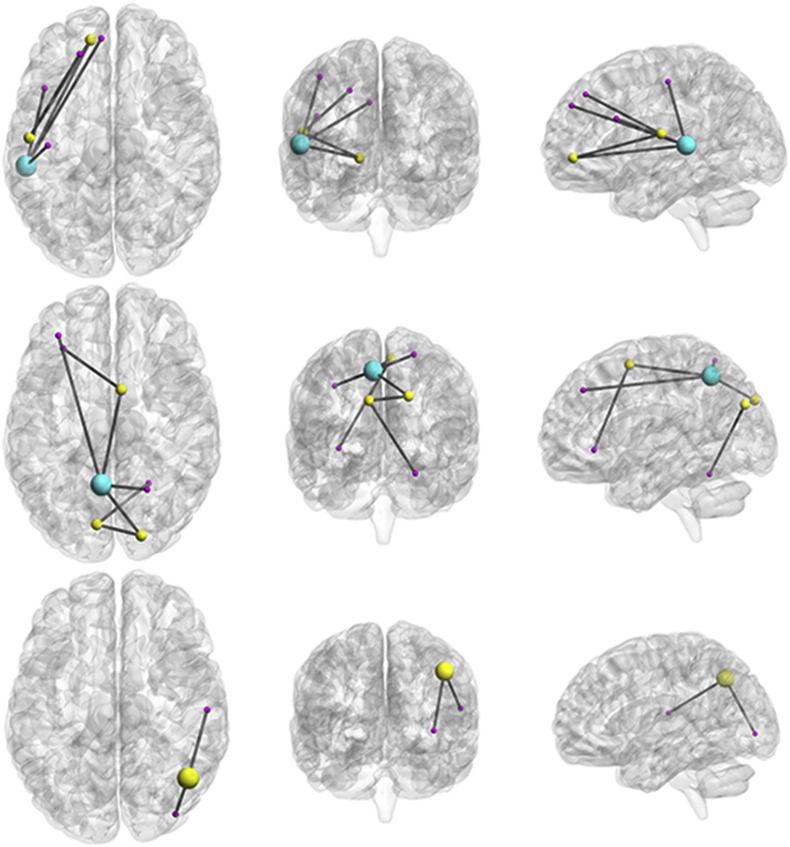
Fig. 1.
Subnetworks with reduced fractional anisotropy (FA) in children with CAS compared to controls represented on brain renderings. Spheres correspond to significant nodes in the analysis. Sphere size is proportional to the number of altered connections originating from that node. The top panel represents subnetwork 1, the middle panel represents subnetwork 2, and the bottom panel represents subnetwork 3. All subnetworks from the left to the right are represented on axial, coronal and sagittal planes, respectively. Sagittal plane is viewed from the left.
Subnetwork 1 comprised 6 left intrahemispheric connections between 7 nodes (p = 0.002) and included connections of frontotemporal regions (dorsolateral, medial and orbital part of the superior frontal gyrus; opercular part of inferior frontal gyrus; superior and middle temporal gyrus) and post-central gyrus. Subnetwork 2 comprised 7 intra- and interhemispheric connections between 8 nodes (p = 0.001) and included intrahemispheric connections between the left precuneus and left middle frontal gyrus and the right superior parietal and right superior occipital gyrus. It also included interhemispheric connections between the left cuneus, left superior occipital gyrus and right cerebellum and interhemispheric connections between the right supplementary motor area, left inferior (orbital part) frontal gyrus and left precuneus. Subnetwork 3 comprised 2 right intrahemispheric connections between 3 nodes (p = 0.036) and included connections between the right angular gyrus, right superior temporal gyrus and right inferior occipital gyrus.
3.3. Relationship between impaired connectivity and clinical measures
The correlation between tract averaged FA of all the significant connections found using NBS and clinical scores for CAS children was assessed. Significant correlations between FA of individual connections and speech/language scores were found in a number of connections, indicating an association between reduction of FA and low speech/language performances (Fig. 2). In subnetwork 1, reduction of FA correlated with low performance in oromotor skills for the connection between the medial part of superior frontal gyrus and middle temporal gyrus (p = 0.02, R = 0.56). Reduction of FA for connections between the opercular part of inferior frontal gyrus and middle temporal gyrus correlated with low diadochokinesis rate (p = 0.01, R = 0.57), with poor expressive grammar (p = 0.02, R = 0.53) and with poor lexical production (p = 0.003, R = 0.67).
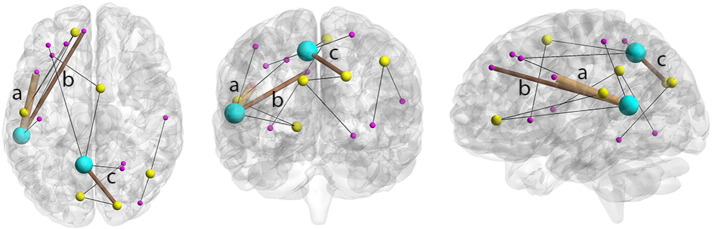
Fig. 2.
Subnetworks that were significantly different between CAS and controls overlaid on a single image, shown on axial, coronal and sagittal planes (sagittal plane shows view from the left). Thin black lines represent edges (connections) whose average FA value does not correlate with any clinical measure. Brown edges represents: a) the connection between the opercular part of the left inferior frontal gyrus and the left middle temporal gyrus (subnetwork 1), whose average FA value correlates with low diadochokinesis rate (p = 0.01, R = 0.57), poor expressive grammar (p = 0.02, R = 0.53) and poor lexical production (p = 0.003, R = 0.67); b) the connection between the medial part of superior frontal gyrus and middle temporal gyrus (subnetwork 1), whose average FA value correlates with oromotor skills (p = 0.02, R = 0.56); c) the connection between the right superior occipital gyrus and left precuneus (subnetwork 2) whose average FA value correlates with low diadochokinesis rate (p = 0.01, R = 0.57).
In subnetwork 2, reduction of FA correlated with low diadochokinesis rate for the intrahemispheric interconnection between the right superior occipital gyrus and left precuneus (p = 0.01, R = 57).
No correlation was found in subnetwork 3.
4. Discussion
The aim of this study was to investigate i) structural connectivity in children with CAS and ii) the relationship between FA within altered connections and speech/language measures.
4.1. Structural connectivity in children with CAS
As expected, the probabilistic whole-brain diffusion tractography showed altered structural connectivity in a number of connections of speech/language networks in children with CAS. Although it has been reported that children with CAS mostly have normal structural brain MRI (Liégeois and Morgan, 2012), studies using advanced quantitative measures of structural MRI in speech disorders revealed the presence of structural brain abnormalities on a microscopic level (Kadis et al., 2014) or morphological abnormalities using voxel based morphometry (Preston et al., 2014). We found reduced FA (i.e. microscopically altered white matter) in a number of language–related connections in the presence of normal neuroanatomical qualitative findings, supporting previous hypotheses on immature or altered development of connectivity in CAS (Preston et al., 2014).
Three subnetworks had altered FA in our subjects with CAS, compared to typically developing children. Two components were intra-hemispheric, one in the right and one in the left hemisphere, and one component had an interhemispheric distribution. This is consistent with previous studies on symptomatic CAS suggesting that a bilateral hemispheric involvement is necessary to result in apraxia of speech in children (Liégeois and Morgan, 2012).
The first subnetwork (subnetwork 1) showing altered connectivity in our children with CAS was centred on the temporal regions of left hemisphere, whose role has been already hypothesized in CAS. For example, advanced imaging using cortical thickness analysis has shown structural changes induced by language training within the posterior superior left temporal gyrus in idiopathic CAS by cortical thickness analysis (Kadis et al., 2014). Also, using voxel-based morphometry, Preston et al. (2014) found bilaterally greater grey matter volume within the superior temporal gyrus in children with residual speech sound errors. Furthermore, the specific involvement of the superior temporal gyrus in CAS is consistent with some of the neural models of speech production, such as the DIVA model (Guenther, 2001). According to this model, the superior temporal gyrus can be considered as a high order auditory cortical region that receives “target” inputs from the premotor cortex to compare them with incoming effective auditory information (Guenther and Hickok, 2015). In our study, altered connectivity in the subnetwork 1 extends to the middle temporal gyrus. A left middle and inferior temporal gyrus hypoactivation pattern on fMRI has been described in child speech sound disorder (Tkach et al., 2011). It has also been demonstrated that both the middle and superior temporal gyri are involved in phonemic discrimination (Ashtari et al., 2004). Likewise, tractography studies demonstrated a wider distribution of fibres in the posterior temporal region than in the classical model, suggesting an extended Wernicke's territory (including the posterior part of both the superior and middle temporal gyri) rather than a localized centre (Catani et al., 2005). Altogether these findings support the hypothesis of structural and functional connections between superior and middle temporal gyrus and their involvement in phonemic discrimination, which could be possibly altered in CAS.
Another node within subnetwork 1 is the inferior frontal gyrus, which has a well-known role in phonological and syntactic processing and has been already proposed to have an evolutionary role in feedback-based articulatory control in humans. Reduced grey matter density in the inferior frontal gyrus has been reported in apraxia of speech due to FOXP2 mutations (Belton et al., 2003). Furthermore, its involvement has been referred by some authors to the mirror neuron system based on the hypothesis that connections between temporal and inferior frontal regions link auditory information to orofacial premotor regions (Catani et al., 2005). Maassen (2002) suggested that a core deficit of CAS could be a reduced capacity to form systemic mappings between articulatory movements and their auditory consequences (Maassen, 2002), a hypothesis that may explain disrupted babbling and early vocal behaviour in all our patients. This symptom has been recently described in a study conducted by our group on a larger sample of CAS children (Chilosi et al., 2015).
Based on our results and previous neuroanatomical studies, temporal-frontal connectivity disruption might represent a possible candidate network for explaining the functional mismatch between auditory feedback and oromotor control in CAS. Moreover, our results showed that the altered frontotemporal network extended to a more posterior connection in the postcentral gyrus (parietal lobe). Although no primary oromotor region was involved, this finding supports a sensory (postcentral) component contribution in the pathogenesis of CAS, as hypothesized in studies on brain lesions (Liégeois et al., 2014) or in theoretical models for CAS, such as the DIVA model (Guenther, 2001).
The second altered subnetwork (subnetwork 2) involved intra- and inter-hemispheric connections, including the left precuneus, right supplementary motor area, left cuneus and right cerebellum. The precuneus has been previously described to have a role in conceptual planning during lexical search (Grande et al., 2012), action initiation (Schmahmann et al., 2008) and is, in general, thought to be directly involved in highly integrated functions (Cavanna and Trimble 2006). The right supplementary motor area has been demonstrated to be related to an anterior-dorsal network associated with fluency in adult nonfluent/agrammatic variant of primary progressive aphasia (Mandelli et al., 2014), and has been shown to be relevant in speech planning and motor and cognitive triggering (Riecker et al., 2005, Nachev et al., 2008). A reduction in grey matter density has been reported in supplementary motor areas, as well as in the abovementioned inferior temporal gyrus, in apraxia of speech due to FOXP2 mutation (Belton et al., 2003). Lastly, the cerebellum has been hypothesized to be involved in CAS by the abovementioned DIVA model, as an alteration in feed-forward mechanisms of speech control (Guenther, 2001, Maassen, 2002). Involvement of both supplementary motor areas and cerebellum agrees with hypotheses on altered motor planning related to speech in CAS (Liégeois et al., 2014, Guenther, 2001).
The third altered subnetwork (subnetwork 3) included intrahemispheric connections between the right angular gyrus, superior temporal gyrus and inferior occipital gyrus. The role of superior temporal gyrus has already been discussed (see above, subnetwork 1); its involvement in the right hemisphere further supports previous hypotheses about bilateral speech/language network involvement in CAS (Liégeois and Morgan, 2012). Involvement of angular gyrus was less expected. The angular gyrus has been described as a critical region for the process of semantic representation (Price et al., 2015, Pallier et al., 2011). Notably, the identified subnetworks 2 and 3 have few nodes in the occipital regions of the brain. However, the regions that showed an altered connectivity were not in the primary visual cortex, but in associative visual regions that have been demonstrated to be involved in semantic processing (Binder et al., 2009, Grande et al., 2012). This explanation is tentative, and implies a semantic network involvement in CAS, as also suggested by the delay in lexical acquisition and no optimal vocabulary skills in the late preschool and early school years. Further studies are required to confirm and interpret these findings.
In summary, as hypothesized, our results have shown distributed altered connectivity in several networks involved in speech/language. They included an “extended” left Wernicke's territory, left postcentral gyrus, right supplementary motor area, cerebellum and right superior temporal gyrus. We have also found that a few regions involved in semantic processing showed a less expected alteration of structural connectivity but their role needs further clarification.
The functional role of these findings still remains to be determined (see below).
4.2. Relationship between FA within altered connections and speech/language measures
Reduced FA correlated with some speech/language measures in children with CAS.
In general, diadochokinesis rate and expressive grammar were more severely impaired in children with CAS, and showed a significant correlation with FA of the altered connection between the middle temporal gyrus and opercular part of the frontal gyrus.
Diadochokinesis has been considered as a useful test for reliably identifying children with CAS (Thoonen et al., 1997) and for detection of neurophysiological mechanisms underlying CAS (Murray et al., 2015). The correlation between diadochokinesis and structural connectivity findings highlights the relevant role of a left-sided network including the middle temporal gyrus and opercular part of the inferior frontal gyrus in determining one of the core symptoms of CAS. Furthermore, lexical production correlated with FA in connections of subnetwork 1, across the left middle temporal gyrus and medial part of the inferior frontal gyrus, supporting the role of a temporal-frontal network in language dysfunction and, possibly, in CAS.
Diadochokinesis also correlated with FA of a connection of subnetwork 2 between right superior occipital gyrus and left precuneus. As both these regions have been assigned an associative role for highly integrated functions (Cavanna and Trimble, 2006, Binder et al., 2009, Grande et al., 2012), we can hypothesize that a complex skill like diadochokinesis requires associative interfaces between speech/language and motor planning and execution. Although its function is largely unknown, the precuneus has been hypothesized to have a role in cognitive functioning, including visuospatial imagery and episodic memory (Cavanna and Trimble 2006), with a possible role in language functioning. To date, there is no evidence for a specific involvement of precuneus in CAS. A confirmation of these exploratory results in future studies is mandatory in order to confirm and clarify hypotheses.
4.3. Limitations
The number of subjects included in this sample was limited due to the low prevalence of the disease and by the collaboration of children required for brain MRI. However, it has been suggested to consider cautiously but positively, significant results in small cohorts (Friston, 2012). Sex unbalance reflects differences in prevalence of CAS (Lewis et al., 2004), however the relatively small number of subjects does not allow for including sex as a covariate in the analysis. Similarly, due to the presence of only two left-handed subjects, the potential effect of brain lateralization has not been considered in the analyses. The age-range of subjects implies different evolutionary stages of CAS. However, all children fulfilled the criteria for CAS at the time of enrolment. Furthermore, we cannot speculate on specificity of our findings to the CAS cohort. We believe that the detailed clinical assessment of these subjects represents a preliminary support to the specificity of our results. As discussed in our previous paper (Chilosi et al., 2015), which included a larger number of subjects, CAS may be considered a multi-level disorder affecting both motor-speech and language competences. It is, however, still unclear whether the co-occurrence of speech-motor and language difficulties, reported also in literature (McNeill et al., 2009, American Speech-Language Hearing Association, 2007, Lewis et al., 2004) can be interpreted as either the effect of a unitary multilevel disorder or as an association of distinct but co-morbid conditions. According to some authors (Terband and Maassen, 2010, Ozanne, 2005, Velleman, 2011), impairment in planning and/or programming spatiotemporal parameters of movement sequences may produce a “cascade” effect that interferes with the early development of phonological, lexical and grammar skills. A further issue refers to the association between verbal and nonverbal oromotor apraxia, an issue that has long been debated in literature (see Ozanne, 2005). In a previous study on a larger sample of children with CAS (Chilosi et al. 2015), measures of nonverbal oromotor skills did not correlate significantly with any specific feature of verbal apraxia. Studies on larger cohorts and enrolment of speech/language disorders other than CAS as control groups will contribute to further qualify our results.
Thirty gradient direction and b value of 1000 are the minimum we consider necessary to apply constrained spherical deconvolution to diffusion data. Nevertheless, greater number of directions and higher b values require longer acquisition times that limit the collaboration of patients and feasibility of the study in a clinical setting.
In conclusion, our findings do not intend to suggest new pathophysiological hypotheses on CAS. Reduction of FA reflects altered white matter microstructure, as the consequence of several factors including axon diameter (Takahashi et al., 2002), lower packing density (Takahashi et al., 2002) or increased membrane permeability (Jones et al., 2013). White matter abnormalities can however be primary, as an altered development of connectivity, or secondary, depending on altered grey matter functioning, thus resulting in either the cause or the consequence of CAS. It is beyond the scope of our current study to speculate on CAS pathogenesis. Instead, we consider our findings as possible biomarkers for CAS, such as an epiphenomenon of complex pathogenic mechanisms that need further investigation.
5. Conclusions
Liégeois and Morgan (2012) reported that 60% of children with CAS have normal structural MRI, but quantitative approaches suggested microscopic abnormalities of white or grey matter (Liégeois et al., 2014). Our data, for the first time, provide evidence of connectivity anomalies in children with CAS across specific brain regions involved in speech/language function. Altered connectivity of both subnetworks 1 and 2 correlated with diadochokinesis as a relevant measure of speech/language dysfunction in children with CAS. We propose altered connectivity as a possible biological marker for CAS, to be considered in the diagnostic approach and possibly to be applied in the monitoring of changes induced by a specific rehabilitative intervention.
Acknowledgements
We would like to acknowledge the children and families who participated in this study, the nursing staff, radiology and administrative staff in the MRI Service and Lab of Stella Maris Scientific Institute. We also acknowledge the Speech Language Pathologist and PROMPT certified instructor Dr. Irina Podda for her contribution in the assessment of some of the children included in the study and Dr. Giuseppe Rossi for his support in the statistical analyses.
This study was funded by PROMPT Institute for Motor Speech Research Grant 2014. The sponsor was not involved in its design, collection, analyses, interpretation of data, and had no role the writing or submission of this paper for publication.
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.nicl.2016.11.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
Relationship between FA within altered connections and speech/language measures.
References
- Ackermann H., Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct. Funct. 2010;214(5–6):419–433. doi: 10.1007/s00429-010-0257-x. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch A., Raznahan A., Bullmore E., Giedd J. The convergence of maturational change and structural covariance in human cortical networks. J. Neurosci. 2013;13(33(7)):2889–2899. doi: 10.1523/JNEUROSCI.3554-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Speech-Language Hearing Association . 2007. Technical Report on Childhood Apraxia of Speech. [Google Scholar]
- Ashtari M., Lencz T., Zuffante P., Bilder R., Clarke T., Diamond A., Kane J., Szeszko P. Left middle temporal gyrus activation during a phonemic discrimination task. Neuroreport. 2004;1(15(3)):389–393. doi: 10.1097/00001756-200403010-00001. [DOI] [PubMed] [Google Scholar]
- Bearzotti F., Fabbro F. Il test per la valutazione delle prassie orofacciali nel bambino. Giornale di Neuropsichatria dell’Età Evolutiva. 2003;23:406–417. [Google Scholar]
- Bello A., Caselli M.C., Pettenati P., Stefanini S. Giunti OS; Firenze: 2010. PinG parole in gioco. [Google Scholar]
- Belton E., Salmond C.H., Watkins K.E., Vargha-Khadem F., Gadian D.G. Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum. Brain Mapp. 2003 Mar;18(3):194–200. doi: 10.1002/hbm.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R., Lange S. Adjusting for multiple testing-when and how? J. Clin. Epidemiol. 2001;54(4):343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzolara D. 1989. Test di vocabolario figurato. Technical Report of the Research Project 500.4/62.1/1134 Supported by a Grant From the Italian Department of Health to IRCCS Stella Maris. [Google Scholar]
- Catani M., Jones D.K., Ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chilosi A.M., Brizzolara D., Lami L., Pizzoli C., Gasperini F., Pecini C., Cipriani P., Zoccolotti P. Reading and spelling disabilities in children with and without a history of early language delay: a neuropsychological and linguistic study. Child Neuropsychol. 2009;15(6):582–604. doi: 10.1080/09297040902927614. [DOI] [PubMed] [Google Scholar]
- Chilosi A.M., Comparini A., Scusa M.F., Orazini L., Forli F., Cipriani P., Berrettini S. A longitudinal study of lexical and grammar development in deaf Italian children provided with early cochlear implantation. Ear Hear. 2013;34(3):e28–e37. doi: 10.1097/AUD.0b013e31827ad687. [DOI] [PubMed] [Google Scholar]
- Chilosi A.M., Lorenzini I., Fiori S., Graziosi V., Rossi G., Pasquariello R., Cipriani P., Cioni G. Behavioral and neurobiological correlates of childhood apraxia of speech in Italian children. Brain Lang. 2015;150:177–185. doi: 10.1016/j.bandl.2015.10.002. 2015 Nov. [DOI] [PubMed] [Google Scholar]
- Cipriani P., Chilosi A.M., Bottari P. Unipress; Padova: 1993. L'acquisizione della morfo-sintassi in italiano-Fasi e processi. [Google Scholar]
- Dronkers N.F. A new brain region for coordinating speech articulation. Nature. 1996;14(384(6605)):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dunn L.M., Dunn L.M. third ed. American Guidance Service Inc., Circle Pines, MN. Italian standardization Omega Edition; 1997. Peabody Picture Vocabulary Test- PPVT. [Google Scholar]
- Friston K. Ten ironic rules for non-statistical reviewers. NeuroImage. 2012;16(61(4)):1300–1310. doi: 10.1016/j.neuroimage.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Grande M., Meffert E., Schoenberger E., Jung S., Frauenrath T., Huber W., Hussmann K., Moormann M., Heim S. From a concept to a word in a syntactically complete sentence: an fMRI study on spontaneous language production in an overt picture description task. NeuroImage. 2012;2(61(3)):702–714. doi: 10.1016/j.neuroimage.2012.03.087. [DOI] [PubMed] [Google Scholar]
- Guenther F.H. Neural Modeling of Speech Production; Proceedings of the 4th International Nijmegen Speech Motor Conference; 2001. [Google Scholar]
- Guenther F.H., Hickok G. Role of the auditory system in speech production. Handb. Clin. Neurol. 2015;129:161–175. doi: 10.1016/B978-0-444-62630-1.00009-3. [DOI] [PubMed] [Google Scholar]
- Hagmann P., Thiran J.P., Meuli R. EPFL; Lausanne: 2005. From Diffusion MRI to Brain Connectomics. [Google Scholar]
- Hillis A.E., Work M., Barker P.B., Jacobs M.A., Breese E.L., Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(Pt 7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Jordan L.C., Hillis A.E. Disorders of speech and language: aphasia, apraxia and dysarthria. Curr. Opin. Neurol. 2006;19(6):580–585. doi: 10.1097/WCO.0b013e3280109260. [DOI] [PubMed] [Google Scholar]
- Kadis D.S., Goshulak D., Namasivayam A., Pukonen M., Kroll R., De Nil L.F., Pang E.W., Lerch J.P. Cortical thickness in children receiving intensive therapy for idiopathic apraxia of speech. Brain Topogr. 2014;27(2):240–247. doi: 10.1007/s10548-013-0308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis J., Koch G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Lerch J.P., Worsley K., Shaw W.P., Greenstein D.K., Lenroot R.K., Giedd J., Evans A.C. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. NeuroImage. 2006;1(31(3)):993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Lewis B.A., Freebairn L.A., Hansen A., Gerry Taylor H., Iyengar S., Shriberg L.D. Family pedigrees of children with suspected childhood apraxia of speech. J. Commun. Disord. 2004;37(2):157–175. doi: 10.1016/j.jcomdis.2003.08.003. Mar–Apr. [DOI] [PubMed] [Google Scholar]
- Liégeois F.J., Morgan A.T. Neural bases of childhood speech disorders: lateralization and plasticity for speech functions during development. Neurosci. Biobehav. Rev. 2012;36(1):439–458. doi: 10.1016/j.neubiorev.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Liégeois F., Mayes A., Morgan A. Neural correlates of developmental speech and language disorders: evidence from neuroimaging. Curr. Dev. Disord. Rep. 2014;7(1):215–227. doi: 10.1007/s40474-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen B. Issues contrasting adult acquired versus developmental apraxia of speech. Semin. Speech Lang. 2002;23(4):257–266. doi: 10.1055/s-2002-35804. [DOI] [PubMed] [Google Scholar]
- McNeill B.C., Gillon G.T., Dodd B. Phonological awareness and early reading development in childhood apraxia of speech (CAS) Int. J. Lang. Commun. Disord. 2009;44(2):175–192. doi: 10.1080/13682820801997353. Mar-Apr. [DOI] [PubMed] [Google Scholar]
- Mandelli M.L., Caverzasi E., Binney R.J., Henry M.L., Lobach I., Block N., Amirbekian B., Dronkers N., Miller B.L., Henry R.G., Gorno-Tempini M.L. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J. Neurosci. 2014;16(34(29)):9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modat M., Ridgway G.R., Taylor Z.A., Lehmann M., Barnes J., Hawkes D.J., Fox N.C., Ourselin S. Fast free-form deformation using graphics processing units. Comput. Methods Prog. Biomed. 2010;98:278–284. doi: 10.1016/j.cmpb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Murray E., McCabe P., Heard R., Ballard K.J. Differential diagnosis of children with suspected childhood apraxia of speech. J. Speech Lang. Hear. Res. 2015;1(58(1)):43–60. doi: 10.1044/2014_JSLHR-S-12-0358. [DOI] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9(11):856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nagao M., Takeda K., Komori T., Isozaki E., Hirai S. Apraxia of speech associated with an infarct in the precentral gyrus of the insula. Neuroradiology. 1999;41(5):356–357. doi: 10.1007/s002340050764. [DOI] [PubMed] [Google Scholar]
- Ogar J., Slama H., Dronkers N., Amici S., Gorno-Tempini M.L. Apraxia of speech: an overview. Neurocase. 2005;1(6):427–432. doi: 10.1080/13554790500263529. [DOI] [PubMed] [Google Scholar]
- Ourselin S., Stefanescu R., Pennec X. Medical Image Computing and Computer-Assisted Intervention (MICCAI). volume 2489 of Lecture Notes in Computer Science. 2002. Robust registration of multi-modal images: towards real-time clinical applications; pp. 140–147. [Google Scholar]
- Ozanne A.E. Childhood apraxia of speech. In: Dodd B., editor. Differential Diagnosis and Treatment of Children with Speech Disorders. second ed. Whurr Pub. Ltd; London: 2005. pp. 71–82. [Google Scholar]
- Pallier C., Devauchelle A.D., Dehaene S. Cortical representation of the constituent structure of sentences. Proc. Natl. Acad. Sci. U. S. A. 2011;8(108(6)):2522–2527. doi: 10.1073/pnas.1018711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannek K., Boyd R.N., Fiori S., Guzzetta A., Rose S.E. Assessment of the structural brain network reveals altered connectivity in children with unilateral cerebral palsy due to periventricular white matter lesions. Neuroimage Clin. 2014;4(5):84–92. doi: 10.1016/j.nicl.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J.L., Molfese P.J., Mencl W.E., Frost S.J., Hoeft F., Fulbright R.K., Landi N., Grigorenko E.L., Seki A., Felsenfeld S., Pugh K.R. Structural brain differences in school-age children with residual speech sound errors. Brain Lang. 2014 Jan;128(1):25–33. doi: 10.1016/j.bandl.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A.R., Bonner M.F., Peelle J.E., Grossman M. Converging evidence for the neuroanatomic basis of combinatorial semantics in the angular gyrus. J. Neurosci. 2015;18(35(7)):3276–3284. doi: 10.1523/JNEUROSCI.3446-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development CoreTeam . R Foundation Statistical Computing; 2008. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Riecker A., Mathiak K., Wildgruber D., Erb M., Hertrich I., Grodd W., Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;2(64(4)):700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Smith E.E., Eichler F.S., Filley C.M. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann. N. Y. Acad. Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg L.D., Potter N.L., Strand E.A. Prevalence and phenotype of childhood apraxia of speech in youth with galactosemia. J. Speech Lang. Hear Res. 2011;54(2):487–519. doi: 10.1044/1092-4388(2010/10-0068). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S1., Yonezawa H., Takahashi J., Kudo M., Inoue T., Tohgi H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurosci. Lett. 2002;25(332(1)):45–48. doi: 10.1016/s0304-3940(02)00914-x. [DOI] [PubMed] [Google Scholar]
- Terband H., Maassen B. Speech motor development in childhood apraxia of speech: generating testable hypotheses by neurocomputational modeling. Folia Phoniatr. Logop. 2010;62(3):134–142. doi: 10.1159/000287212. [DOI] [PubMed] [Google Scholar]
- Thoonen G., Maassen B., Gabreëls F., Schreuder R., de Swart B. Towards a standardised assessment procedure for developmental apraxia of speech. Eur. J. Disord. Commun. 1997;32(1):37–60. doi: 10.3109/13682829709021455. [DOI] [PubMed] [Google Scholar]
- Tournier J.D., Yeh C.H., Calamante F., Cho K.H., Connelly A., Lin C.P. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. NeuroImage. 2008;15(42(2)):617–625. doi: 10.1016/j.neuroimage.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Tkach J.A., Chen X., Freebairn L.A., Schmithorst V.J., Holland S.K., Lewis B.A. Neural correlates of phonological processing in speech sound disorder: a functional magnetic resonance imaging study. Brain Lang. 2011;119(1):42–49. doi: 10.1016/j.bandl.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Velleman S.L. Lexical and phonological development in children with childhood apraxia of speech. A commentary on Stoel-Gammon's ‘relationships between lexical and phonological development in young children’. Journal of Child Language. 2011;38(1):82–86. doi: 10.1017/S0305000910000498. [DOI] [PubMed] [Google Scholar]
- Vicari S., Marotta L., Luci A. Trento; Ed Erickson: 2007. Test TFL - Test Fono Lessicale Valutazione delle abilità lessicali in età prescolare. [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between FA within altered connections and speech/language measures.