Abstract
Background
The contribution of fat loss versus muscle wasting to the loss of body weight seen in hyperthyroid cats is unknown.
Objectives
To investigate body weight, body condition score (BCS), and muscle condition score (MCS) in hyperthyroid cats.
Animals
Four hundred sixty‐two cats with untreated hyperthyroidism, 117 of which were reevaluated after treatment.
Methods
Prospective cross‐sectional and before–after studies. Untreated hyperthyroid cats had body composition evaluated (body weight, BCS, and MCS). A subset of these cats were reevaluated 3–12 months after treatment when euthyroid.
Results
Pretreatment body weight (median, 4.36 kg; IQR, 3.5 to 5.2 kg) was lower than premorbid weight (5.45 kg; IQR, 4.6 to 6.4 kg, P < .0001) recorded 1–2 years before diagnosis. 154 (35.3%) cats were thin or emaciated; 357 (77.3%) had loss of muscle mass. Cats showed increases in body weight (median, 4.1 kg to 5.0 kg), BCS (median, 3/5 to 3.5/5), and MCS (2/3 to 3/3) after treatment (P < .001), but mild‐to‐moderate muscle wasting persisted in 45% of treated cats.
Conclusions and Clinical Importance
Most hyperthyroid cats lose body weight but maintain an ideal or overweight BCS, with only a third being underweight. As in human hyperthyroid patients, this weight loss is associated with muscle wasting, which affects >75% of hyperthyroid cats. Successful treatment leads to weight gain and increase of BCS in most cats, but almost half fail to regain normal muscle mass.
Keywords: Body composition, Dietary protein, Feline, Muscle condition, Sarcopenia, Thyroid gland
Abbreviations
- BCS
body condition score
- MCS
muscle condition score
- T4
thyroxine
- T3
triiodothyronine
- fT4
free T4
- TSH
thyroid‐stimulating hormone
- IQR
interquartile range
- CKD
chronic kidney disease
Hyperthyroidism is a catabolic state associated with increased energy expenditure,1, 2 increased lipolysis,3, 4 and increased protein turnover.5, 6 These metabolic effects commonly lead to loss of body weight associated with a decrease in both fat stores and lean body mass. In untreated hyperthyroid human patients, initial weight loss is predominantly caused by a decrease in lean body mass (primarily muscle), rather than loss of fat.7, 8, 9 After successful treatment, lost muscle mass is restored first, before that of the fat deposits.7, 9, 10, 11, 12, 13
In hyperthyroid cats, weight loss has long been recognized as a classical feature of the disease, but muscle wasting is only rarely mentioned as a clinical feature of feline hyperthyroidism.14, 15 Furthermore, these descriptions are based on early studies of feline hyperthyroidism, in which most cats were very thin to emaciated.16, 17, 18 Today, however, clinicians diagnose hyperthyroid cats at an early or mild stage of disease, with only minimal weight loss or other clinical signs, before being treated.14, 19 If these mildly affected cats are similar to humans, preferential loss of muscle mass over fat mass might be expected. However, the contribution of fat and muscle wasting to the overall loss of body weight in hyperthyroid cats is not known.
Our aim in this study was to investigate the body weight, body condition score (BCS), and muscle condition score (MCS) in a large population of cats with untreated hyperthyroidism to determine the prevalence of an underweight condition and muscle loss in this disease. Our second aim was to prospectively follow‐up a subset of these cats to examine the effects of successful radioiodine treatment on their body weights, as well as their body and muscle condition scores.
Materials and Methods
Selection of Animals and Study Design
This study was conducted in 2 parts. The first was a prospective cross‐sectional study conducted from June 2013 to December 2015, and included hyperthyroid cats referred to the Animal Endocrine Clinic for evaluation before radioiodine treatment. The second was a before–after study20, 21 involving a subset of the cats from the initial study that returned for reevaluation 3–12 months after treatment with radioiodine.
Initial Cross‐Sectional Study
To be eligible for inclusion, hyperthyroid cats had to undergo a thorough evaluation that included review of the past medical record, detailed owner medical and dietary history, complete physical examination, routine laboratory testing (CBC and serum biochemical profile), and determination of serum thyroid panel (thyroxine [T4], triiodothyronine [T3], free T4 [fT4], and thyroid‐stimulating hormone [TSH]).22, 23 All study cats also underwent quantitative thyroid scintigraphy, which was used as the reference standard to confirm hyperthyroidism.23, 24, 25
Cats were ineligible for inclusion if their hyperthyroidism had been well controlled, as demonstrated by normalization of serum T4 concentration with methimazole or a low‐iodine diet within the last 3 months, if concurrent nonthyroidal disease, such as azotemia, was detected, if no premorbid weight was available, or if the diagnosis was not confirmed with quantitative scintigraphy. Cats that were resistant or refractory to the effects of methimazole, with persistent and generally severe hyperthyroidism, were not excluded from this study.
On the day of treatment with radioiodine, body weight was determined to 0.05 kg with a calibrated digital scale,1 which was regularly validated for accuracy with test weights (2, 4, and 8 kg). This hyperthyroid body weight was compared to the last known premorbid body weight, extracted from the referring veterinarian's or owner's record. Each cat was then assigned a BCS by 2 investigators (MEP and CAC) who independently scored each cat with a 5‐point system (1 = emaciated; 2 = too thin; 3 = ideal weight; 4 = too fat; 5 = obese),26, 27, 28 with results averaged. Finally, each cat was also assigned a MCS with a 4‐point system (0 = severe muscle wasting; 1 = moderate wasting; 2 = mild wasting; 3 = normal muscle mass).28, 29, 30 This MCS evaluation includes visual examination and palpation of muscle mass over the spine, scapulae, skull, and wings of the ilia, as previously described.28, 29, 30
Before–after Study
All owners of cats enrolled in the initial cross‐sectional study were encouraged to return to the Animal Endocrine Clinic for a recheck examination at 3–6 months after treatment with radioactive iodine. Only cats available for recheck were included in this before‐and‐after study. All of these cats again underwent a thorough evaluation, which included a review of the medical and dietary history since time of 131I treatment, complete physical examination, serum biochemical testing (including creatinine and urea nitrogen), and determination of serum T4 and fT4 concentrations. All cats were carefully reweighed and then had BCS and MCS again assigned by the same 2 investigators who independently scored each cat without knowledge of the cats' pretreatment BCS, MCS, or response to 131I treatment.
Based on results of post‐treatment thyroid testing, we classified cats as euthyroid (defined as normal serum T4 and fT4 concentrations) or persistently hyperthyroid (high serum T4 and fT4 concentrations). Cats were ineligible for inclusion in this part of the study if they had low serum T4 and fT4 concentrations (consistent with overt hypothyroidism).
Data and Statistical Analyses
All statistical analyses were performed by proprietary statistical software.2 , 3 Data were assessed for normality by the D'Agostino–Pearson test and by visual inspection of graphical plots.31 Data were not normally distributed; therefore, all analyses used nonparametric tests.32 Results are reported as median (IQR, 25th–75th percentile) and are represented graphically as box‐and‐whisker plots and bar graphs. For all analyses, statistical significance was defined as P < .05.
For analysis, the untreated hyperthyroid cats were further categorized into 3 equal‐sized groups (quantiles) of disease severity based on total T4 concentration (i.e, mild, moderate, and severe disease).33 The cats were also divided into 3 groups based upon their age stage (i.e, mature [7–10 years], senior [11–14 years], or geriatric [≥15 years]).34, 35 Finally, the cats were divided into 4 groups based upon the type of diet fed (i.e, only canned or wet commercial cat food; only dry commercial cat food; both canned and dry food; or home‐prepared or raw meat).
Continuous variables were compared between groups by the Mann–Whitney U or Kruskal–Wallis tests; comparisons between 2 or more measurements within a group (before–after) were compared with a Wilcoxon signed ranks or Friedman test,32 followed by the Dunn's multiple comparisons test.36 Categorical variables were compared among groups by the chi‐square test (or Fisher's exact test, where appropriate) and within a group for correlated proportions (before‐after) by the McNemar's test.37 Correlation testing was performed by Spearman rank correlation coefficient.
Results
Study 1 (Cross‐Sectional Study, Untreated Hyperthyroid Cats)
Signalment, Clinical, and Laboratory Findings
During the 2.5‐year study period, we evaluated 786 hyperthyroid cats, of which 462 met the eligibility requirements (Fig 1). The 462 study cats ranged in age from 7 to 18 years (median, 13 years; IQR, 11 to 14 years). When divided into 3 groups based on age stage, 91 cats (20%) were mature, 275 (60%) were senior, and 96 (21%) were geriatric.
Figure 1.
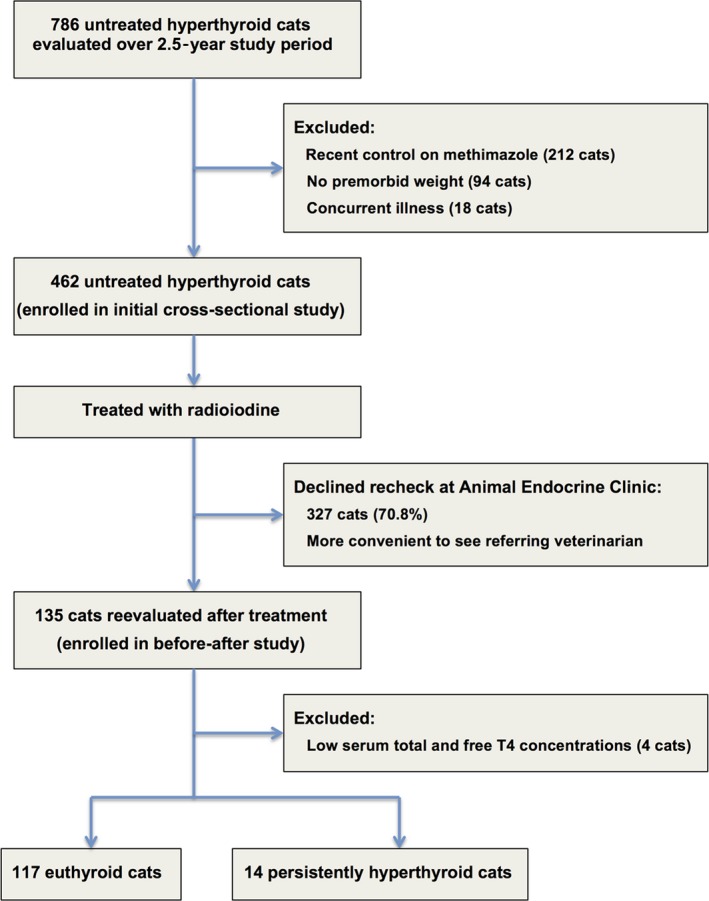
Flowchart for enrollment of hyperthyroid cats into study 1 (cross‐sectional study of untreated cats) and study 2 (before–after treatment).
Breeds included domestic longhair and shorthair (n = 413; 89%), Siamese (17), Maine Coon (12), Persian (3), Scottish Fold (3), Burmese (2), Norwegian Forest Cat (2), and one cat of each of 10 other breeds (Abyssinian, American Curl, Bengal, Bombay, Devon Rex, Korat, Oriental, Ragdoll, Russian Blue, and Tonkinese). Of these, 236 (51.1%) were male and 226 were female; all had been neutered.
Common historical signs reported of these cats included weight loss despite an increased appetite, vomiting, increased activity, and polydipsia/polyuria (Table 1). Diet fed consisted of a variety of both moist and dry commercial cat foods to 298 (65%), moist only to 110 (24%), dry only to 46 (10%), and home‐prepared or raw meat diet to 8 (1.7%) cats. On physical examination, the most frequent findings included palpable enlargement of one or both thyroid lobes, muscle wasting, and thin body condition.
Table 1.
Common historical signs, physical examination, and laboratory findings in 462 cats with untreated hyperthyroidism
| Finding | Number of Cats | Percent of Cats (%) |
|---|---|---|
| Historical signs: | ||
| Weight loss | 425 | 92.0 |
| Increased appetite | 253 | 54.8 |
| Vomiting | 216 | 46.8 |
| Hyperactive | 190 | 41.1 |
| Polydipsia/polyuria | 152 | 32.9 |
| Diarrhea/increased fecal volume | 95 | 20.6 |
| Decreased activity | 60 | 13.0 |
| Heat intolerance | 59 | 12.8 |
| Physical examination findings: | ||
| Palpable thyroid nodule | 450 | 97.5 |
| Muscle wasting | 356 | 77.1 |
| Thin body condition | 160 | 34.6 |
| Dental disease | 206 | 44.6 |
| Tachycardia (≥240 bpm) | 145 | 31.4 |
| Cardiac murmur | 134 | 29.0 |
| Laboratory findings: | ||
| High alanine aminotransferase | 261 | 56.5 |
| High alkaline phosphatase | 107 | 23.2 |
| High aspartate aminotransferase | 52 | 11.3 |
| High hematocrit (PCV) | 50 | 10.8 |
| High serum T4 | 444 | 96.1 |
| High serum fT4 | 450 | 97.4 |
| High serum T3 | 281 | 60.1 |
| Low serum TSH | 453 | 98.1 |
The most common abnormalities detected on routine laboratory testing were high levels of serum alanine transferase (median, 121 U/L; reference interval [RI], 10–100 U/L) and alkaline phosphatase (median, 57 U/L; RI, 10–100 U/L) activities (Table 1). Median serum concentrations of T4, T3, and fT4 were high, whereas the median serum TSH concentration was undetectable (Table 2). Almost all cats had high serum concentrations of T4 and fT4, together with undetected TSH concentrations (Table 1). When subdivided into 3 equal‐sized groups (154 each) based on the magnitude of the cats' serum T4 concentrations, the mild disease group had serum T4 concentrations ≤7.2 μg/dL, the moderate group had T4 concentrations ranging from 7.3 to 11.4 μg/dL, and the severe group had serum T4 concentrations ≥11.5 μg/dL.
Table 2.
Median (IQR) serum concentrations of T4, T3, fT4, and TSH in 462 cats with untreated hyperthyroidism (divided into 3 quantiles of disease severity based on T4 concentrations)
| Cat Group | Serum T4 (μg/dL) | Serum T3 (ng/dL) | Serum fT4 (pmol/L) | Serum TSH (ng/mL) |
|---|---|---|---|---|
| Hyperthyroid (462) | 8.7 (6.5–12.9) | 139 (89–224) | 80 (68–100) | <0.03 (<0.03–<0.03) |
| Mild (154) | 5.7 (4.8–6.5) | 76 (59–90) | 64 (57–69) | <0.03 (<0.03–<0.03) |
| Moderate (154) | 8.7 (7.8–10.0) | 141 (123–167) | 81 (78–94) | <0.03 (<0.03–<0.03) |
| Severe (154) | 16.6 (12.9–20.9) | 284 (232–372) | 100 (100–100) | <0.03 (<0.03–<0.03) |
| Clinically normal (131) | 2.1 (1.7–2.6) | 49 (40–60) | 32 (26–38) | 0.04 (<0.03–0.07) |
Reference intervals: T4 = 0.9–3.8 μg/dL; T3 = 35–120 ng/dL; fT4 = 10–51 pmol/L; and cTSH = <0.03–0.3 ng/mL.
Body Weight
The 462 untreated hyperthyroid cats weighed less (median, 4.36 kg; IQR, 3.5–5.2 kg) than they had 1–2 years before diagnosis of hyperthyroidism (5.45 kg; IQR, 4.6–6.4 kg; P < .0001). The most severely hyperthyroid cats weighed less (4.1 kg) than the cats with mild or moderate hyperthyroidism (4.6 kg or 4.4 kg; P = .025).
Similarly, geriatric cats weighed less (median, 3.7 kg) than did the senior or mature cats (4.4 kg or 4.5 kg; P = .0003). This difference in body weight between the 3 age groups was unrelated to severity of hyperthyroid disease because the serum T4 concentrations in the lighter geriatric cats (7.9 μg/dL) was significantly lower than concentrations in the heavier senior or mature cats (8.6 μg/dL or 10.1 μg/dL; P = .022). Finally, diet type (canned, dry, both canned and dry, or home‐made or raw) had no detectable effect on bodyweight (P = .25).
Body Condition Scores
Of the 462 cats with untreated hyperthyroidism, 160 (35%) had a low BCS (i.e, thin to emaciated), 228 (49%) had an ideal BCS, and 74 (16%) had a high BCS (too fat to obese). Serum T4 correlated negatively (but weakly) with BCS (r = −0.152; P < .001). When the 462 cats were subdivided into 3 groups of disease severity based on the magnitude of the serum T4 value, prevalence of low BCS (too thin) increased with severity of disease (P = .008, Fig 2A).
Figure 2.
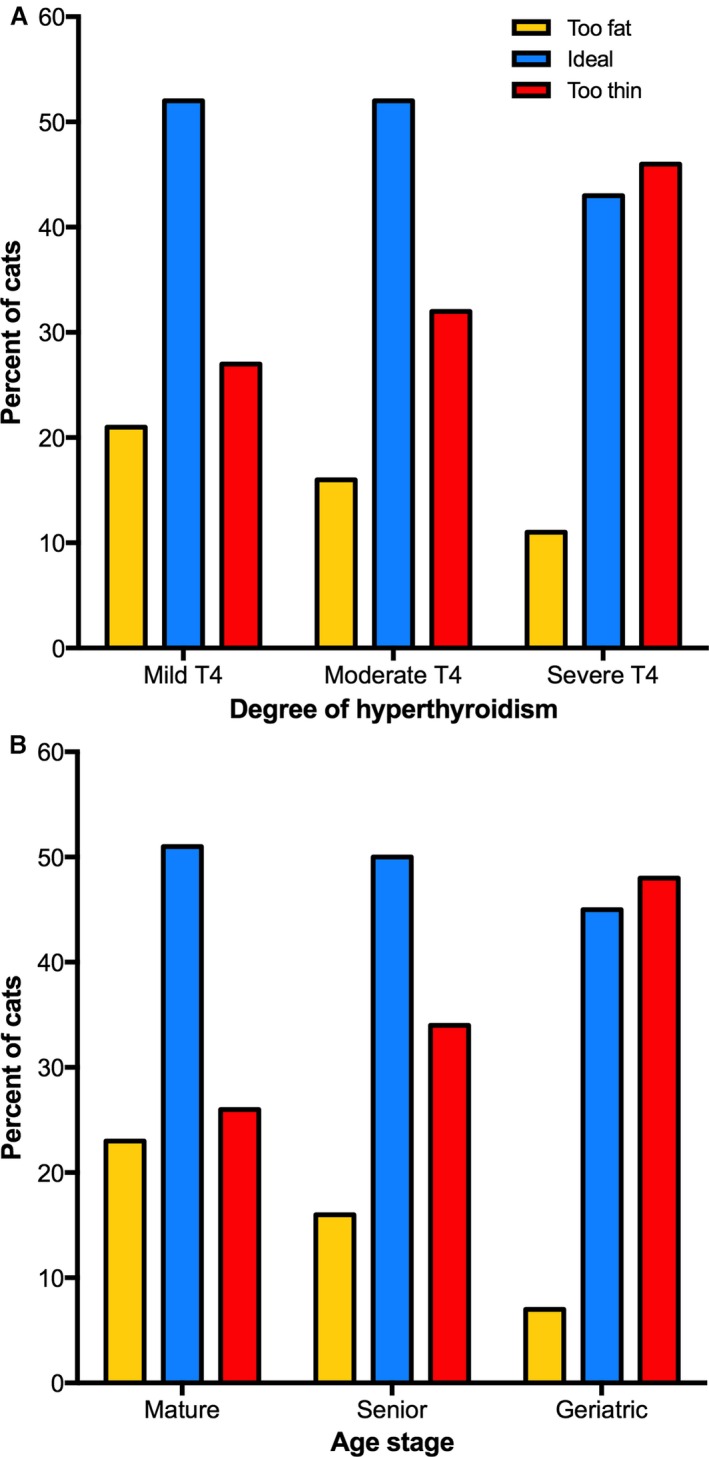
(A) Bar graphs depicting the BSCs of the 462 untreated hyperthyroid cats categorized into 3 equal‐sized groups (n = 154) of disease severity based on total T4 concentration (i.e, mild, moderate, and severe disease). In each of the 3 groups, the percentage of hyperthyroid cats with low (too thin), ideal, and high (too fat) BCSs is depicted. As the severity of hyperthyroid disease increases, notice that the prevalence of too‐fat and ideal cats decreases, whereas prevalence of thin cats increases (P = .008). (B) Bar graphs depicting the BSCs of the 462 untreated hyperthyroid cats categorized into 3 groups based on their life stage (mature, senior, or geriatric). As the age stage of the cats increases from mature to geriatric, notice that the prevalence of fat cats decreases, whereas the prevalence of thin cats increases (P = .0087).
Age also correlated negatively and weakly with BCS (r = −0.247, P < .0001). When the 462 cats were subdivided into 3 groups based on their life stage (mature, senior, or geriatric), prevalence of low BCS was significantly higher in geriatric cats than the senior or mature groups (P = .009; Fig 2B). However, diet type (canned, dry, both canned and dry, or home‐made or raw) had no association with prevalence of any BCS (P = .21).
Muscle Condition Scores
Of the 462 cats with untreated hyperthyroidism, 105 (23%) had a normal MCS, whereas mild, moderate, and severe muscle loss was recorded in 176 (38%) 140 (30%), and 41 (9%), respectively. Serum T4 concentrations correlated negatively but weakly with MCS (r = −0.156, P = .008). When the 462 cats were subdivided into 3 groups based on the magnitude of the serum T4 value, the prevalence of moderate to severe muscle wasting increased with increasing severity of disease (P = .0002; Fig 3A).
Figure 3.
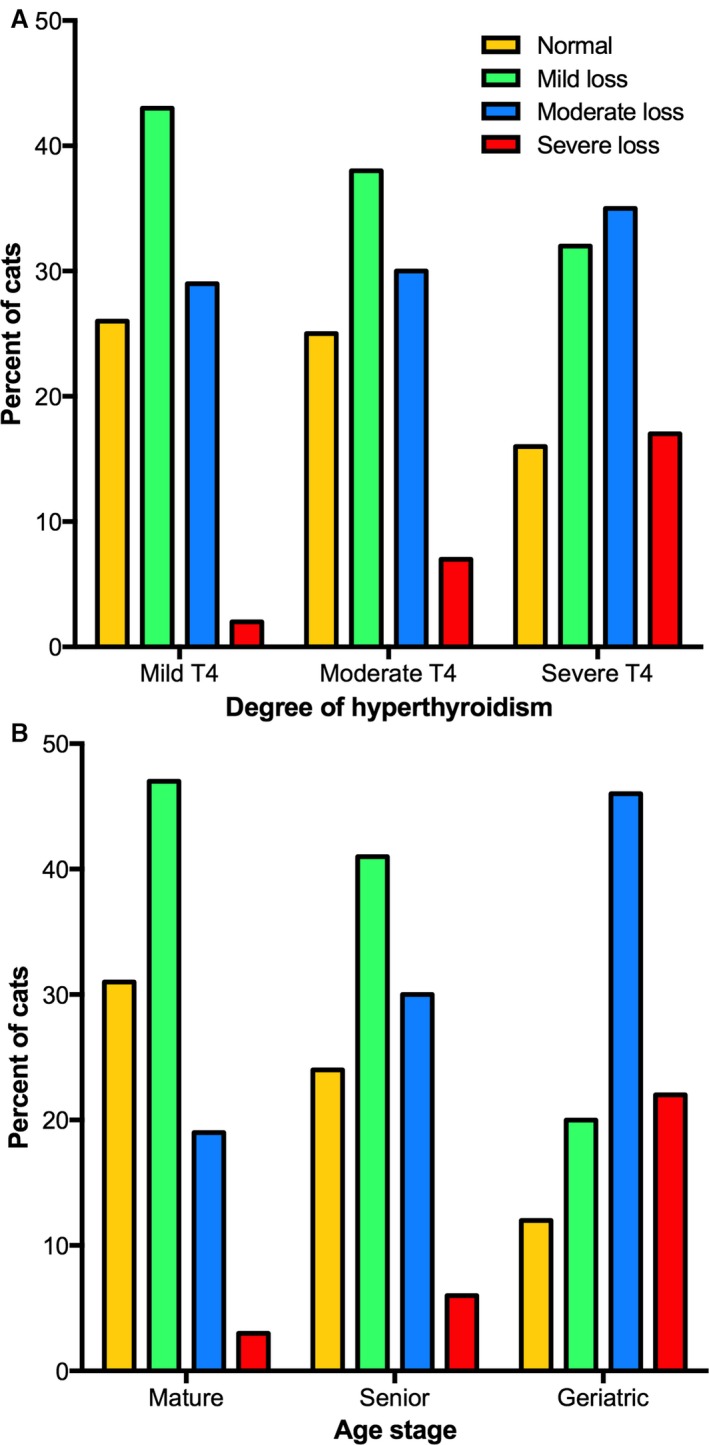
(A) Bar graphs depicting the MCSs of the 462 untreated hyperthyroid cats categorized into 3 equal‐sized groups (n = 154) of disease severity (i.e, mild, moderate, and severe disease). In each of the 3 groups, the percentage of hyperthyroid cats with normal muscle mass, and mild, moderate, and severe muscle loss is depicted. As the severity of hyperthyroid disease increases, notice that the prevalence of normal muscle mass cats decreases, whereas the prevalence of moderate to severe muscle wasting increases (P = .0002). (B) Bar graphs depicting the MCSs of the 462 untreated hyperthyroid cats categorized into 3 groups based on their life stage (mature, senior, or geriatric). As the age stage of the cats increases from mature to geriatric, notice that the prevalence of normal muscle mass decreases, whereas the prevalence of moderate and severe muscle wasting increases (P < .0001).
Similarly, age correlated negatively but weakly with MCS (r = −0.229; P < .001). When the 462 cats were subdivided into 3 groups based on their life stage (mature, senior, or geriatric), prevalence of moderate to severe muscle wasting was higher in geriatric cats than the younger groups (P < .0001; Fig 3B). However, diet type showed no association with the prevalence of normal or low MCS (P = .42).
Study 2 (Hyperthyroid Cats, Before‐and‐After Treatment with Radioiodine)
Signalment and Serum Thyroid Hormone Findings
We were able to revaluate 135 hyperthyroid cats 3–12 months after radioiodine treatment, of which 131 met the eligible requirements for this study (Fig 1). Of these, 117 were euthyroid and 14 were persistently hyperthyroid.
The 117 euthyroid cats ranged in age from 7 to 18 years (median, 12 years; IQR, 10–14 years). When these 117 cats were divided into 3 groups based on age stage, 30 cats were mature, 65 were senior, and 22 were geriatric. Breeds included domestic longhair and shorthair (109 cats), Siamese (2 cats), Maine Coon (2 cats), and 1 cat each of the following breeds (American Curl, Bombay, Devon Rex, Persian). Of these, 62 (53%) were female and 55 were male; all had been neutered. When these 117 cats were subdivided based on their severity of disease (when untreated), hyperthyroidism was mild (≤7.2 μg/dL) in 37, moderate in 38, and severe (≥11.5 μg/dL) in 42 cats. When reevaluated after treatment, the median serum T4 concentration had decreased from 9.2 μg/dL to 1.7 μg/dL, whereas the fT4 concentration had decreased from 95 pmol/L to 17 pmol/L (P < .0001). At the time of follow‐up evaluation, serum concentrations of T4 and fT4 were within reference intervals in all 117 of these euthyroid cats.
The 14 cats with persistent hyperthyroidism ranged in age from 10 to 20 years (median, 14.5 years; IQR, 11 to 17 years). All were domestic longhair or shorthair. Of these, 7 were female and 7 were male. When these 14 cats were subdivided based on their severity of disease (when untreated), hyperthyroidism was mild in 2, moderate in 4, and severe in 8 cats. When reevaluated after treatment, the median serum T4 concentration had decreased from 13.2 μg/dL to 5.9 μg/dL, whereas the fT4 concentration had decreased from 100 pmol/L to 64 pmol/L (P = .002). However, post‐treatment serum concentrations of T4 and fT4 remained high in all 14 of these cats with persistent hyperthyroidism.
Body Weight
In the 117 euthyroid cats reevaluated 91–358 days (median, 204 days; IQR, 178–234 days) after treatment, median body weight increased from 4.1 kg (IQR, 3.5–5.2 kg) to 5.0 kg (IQR, 4.1–6.1 kg) (P < .0001; Fig 4), with a median weight gain of 0.6 kg (IQR, 0.4–1.0 kg; range, 0.0–3.0 kg). Fifty‐three (45%) of the 117 cats regained >90% of the weight lost when hyperthyroid; only 4 cats (3.4%) showed <15% weight gain. Although higher than the hyperthyroid weight, the median post‐treatment body weight remained lower than premorbid body weight (5.3 kg; IQR, 4.3–6.3 kg; P < .001) (Fig 4).
Figure 4.
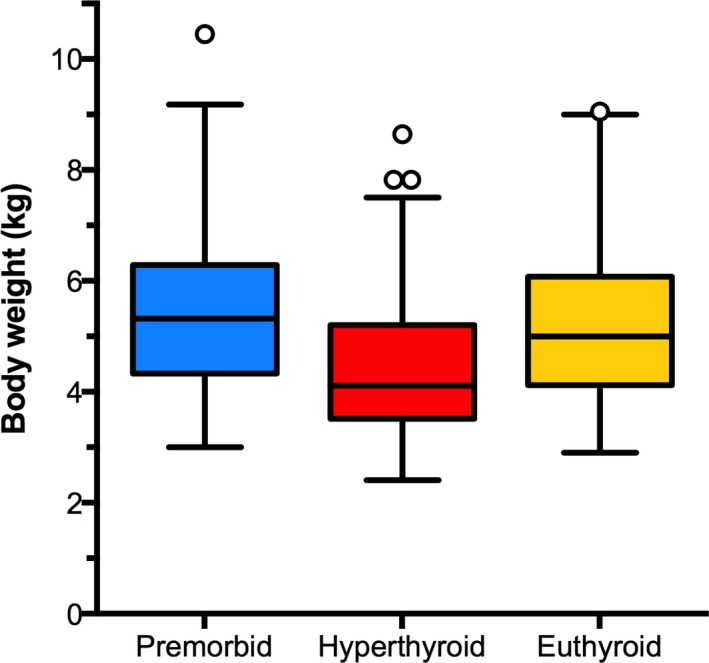
Box plots of premorbid, hyperthyroid, and post‐treatment (euthyroid) body weights in 117 cats evaluated before and after the successful treatment with radioiodine. Notice that the cats' premorbid body weight fell significantly (P < .0001) when hyperthyroid, whereas the post‐treatment, euthyroid weight increased significantly (P < .001) to levels similar to premorbid weight.
In contrast to the euthyroid cats, the 14 cats with persistent hyperthyroidism showed no significant change in median body weight (4.2 kg vs. 3.9 kg; P = .14) when reevaluated 70–280 days (median, 171 days) after treatment with radioiodine. Only 3 of the 14 cats showed >15% weight gain, and none reached their premorbid body weight.
Body Condition Scores
In the 117 euthyroid cats reevaluated after treatment, BCS increased after treatment (P < .001). After treatment, the proportion of cats having an ideal or high BCS increased as those with low BCS decreased (P < .001; Fig 5A). Of 43 cats (37%) that were considered too thin at time of hyperthyroidism, 37 increased BCS (to ideal BCS in 31), whereas 5 failed to increase BCS and remained thin despite a slight weight gain (0.15, 0.3, 0.5, 0.5, and 0.65 kg, respectively). Of the 11 (9%) euthyroid cats that remained thin after treatment, overt chronic kidney disease (CKD) was detected in 7 (serum creatinine concentrations, 2.2–3.4 mg/dL). The median age of the cats that had persistent low BCSs was significantly higher than the age of the cats with normal or high BSCs (14 vs. 12 years; P = .016). However, there was no significant difference between the prevalence of mature, senior, or geriatric age stage (P = .15) or type of diet fed (P = .15) in cats with normal or high BSCs vs those with persistent low BCSs.
Figure 5.
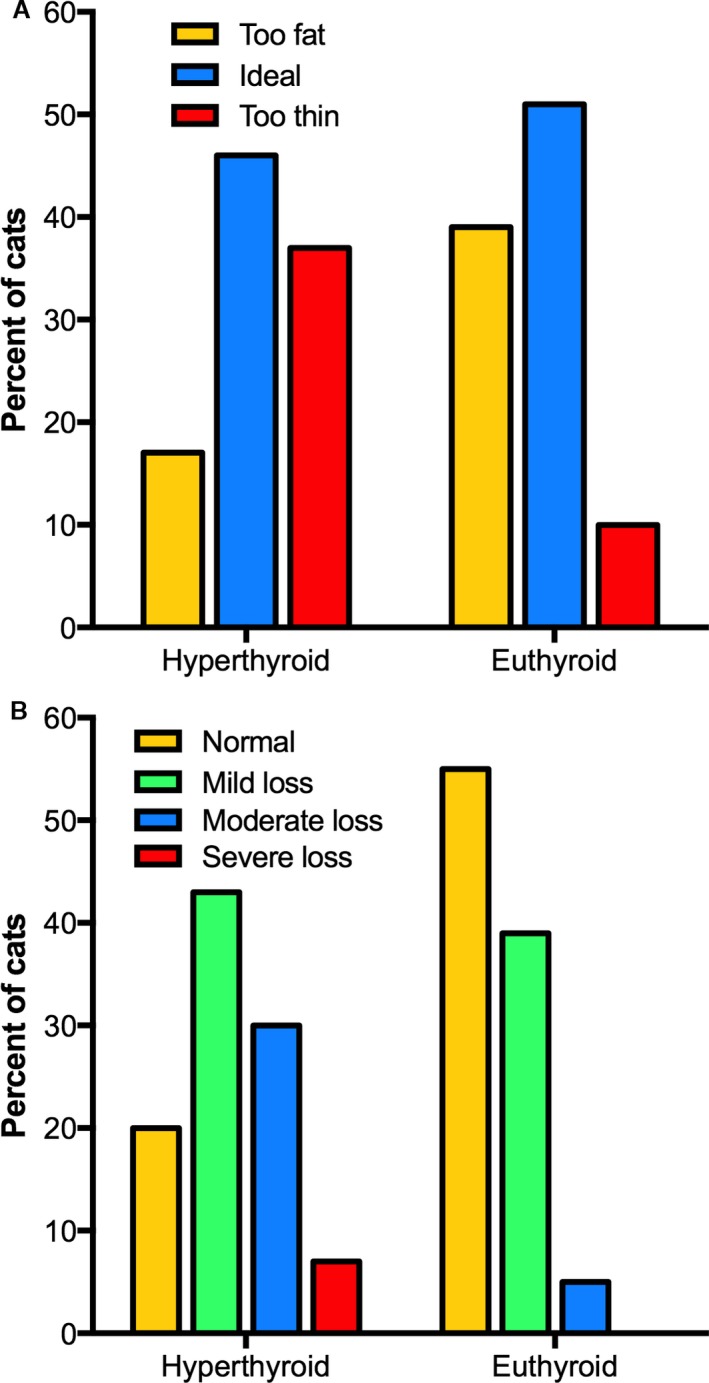
Before and after BCSs and MCSs of 117 cats reevaluated after successful treatment with radioiodine. (A) Bar graphs depicting the percentage of cats with low (too thin), ideal, and high (too fat) body condition scores. After treatment, notice that the prevalence of normal and overweight cats increased, whereas underweight cats decreased (P < .001). (B) Bar graphs depicting the percentage of cats with normal muscle mass, and mild, moderate, and severe muscle wasting. After treatment, notice that the prevalence of normal muscle mass increased, whereas mild, moderate, and severe muscle wasting decreased (P < .001). Severe muscle wasting did not persist in any of the cats after treatment.
In contrast to the euthyroid cats, the 14 cats with persistent hyperthyroidism showed no significant change (P = .5) in median BCS after treatment with radioiodine. All 7 cats that were too thin at time of initial revaluation remained thin after treatment. In addition, 1 cat that had an ideal BCS on initial evaluation developed a low BCS after treatment.
Muscle Condition Scores
In the 117 euthyroid cats reevaluated after treatment, MCS increased significantly (P < .001). After treatment, the number of cats having a normal MCS increased, whereas the number of cats with muscle wasting decreased (P < .001; Fig 5B). Of the 117 cats, 93 (80%) cats originally had a low MCS; after treatment, MCS normalized in 42 (45%) of the 93 cats, increased but remained low in 35, and showed no change in 16. Of the 51 euthyroid cats that remained muscle wasted after treatment, overt CKD was detected in 17. Of the 93 cats that had originally had a low MCS, there was no difference in median follow‐up times between the 42 treated cats that normalized muscle mass and the 51 cats with persistent muscle wasting (203 vs 210 days; P = .36).
Compared to the 67 treated cats with normal MCSs, the cats with persistent muscle wasting were older (13 vs 12 years; P < .01) and had a higher proportion of senior and geriatric age stages (P < .0001). However, there was no significant difference (P = .34) between the types of diet fed to cats with normal or low MCSs.
In contrast to the euthyroid cats, the 14 cats with persistent hyperthyroidism showed no significant change (P = .5) in median MCS after treatment with radioiodine. These 14 cats had mild (n = 6) or moderate (n = 8) muscle wasting at time of initial revaluation, and all had persistent muscle wasting after treatment, with no improvement in MCS.
Discussion
Our results indicate that most hyperthyroid cats lose body weight but maintain an ideal or high (overweight) BCS, with only a third being too thin. As in human hyperthyroid patients, this weight loss appears to be largely because of muscle wasting, affecting >75% of hyperthyroid cats. Resolution of hyperthyroidism leads to weight gain and increased BCS in most cats, but many fail to completely regain normal muscle mass. Severe hyperthyroidism and geriatric age both appear to contribute independently to the prevalence of low BCSs and MCSs in these cats.
Our findings are similar to those observed in human hyperthyroid patients. Skeletal muscle is an important target of thyroid hormone action.38 In humans and experimental animals, hyperthyroidism accelerates whole body protein turnover and catabolizes muscle tissue.5, 39, 40, 41, 42, 43 This leads to loss of muscle mass, which has long been recognized as an important clinical feature in human hyperthyroid patients.44, 45, 46 In fact, human hyperthyroid patients lose body weight mainly caused by loss of muscle mass, with loss of fat mass playing only a modest role.7, 9, 10, 11, 12, 13 After treatment, weight is regained primarily by restoring lost muscle mass, together with a lesser gain in fat mass.7, 9, 10, 11, 12, 13 In hyperthyroid cats, loss of muscle mass may also be of greater importance than fat loss, especially in the earlier stages of the disease. The importance of muscle wasting to overall weight loss was not well appreciated in early publications of feline hyperthyroidism,16, 17, 18 probably because cats of those reports generally had more chronic and severe disease in which marked loss of fat as well as muscle mass would be expected.
Hyperthyroid cats are currently being diagnosed at an earlier and milder stage than they were over two decades ago when we last reported an update on the prevalence of clinical and laboratory findings in untreated hyperthyroid cats examined in the New York City area.47 Weight loss remains a very common sign (92%), but the prevalence of thinness (low BCS) has essentially halved, from 65% in 1993 to only 35% in this study.47 In fact, a considerable number of hyperthyroid cats diagnosed today are overweight or even obese (16% in the present series), a finding never reported in earlier case series.16, 17, 18, 47
Clinically, body weight is a precise, repeatable, and objective measurement.48, 49 When body weight is monitored regularly over months to years, it is a highly sensitive measure (especially when the same calibrated scale is consistently used), allowing one to detect even slight weight loss (e.g, as cats develop early or mild hyperthyroidism). For a primary care practitioner who lacks confidence in performing body condition scoring (which is both subjective and imprecise), or for a multidoctor hospital in which different veterinarians examine a cat over time, use of body weight as an early warning indicator for health status is arguably the best approach. Finding subtle changes in body weight can alert the clinician to take a closer look (especially at BCS and MCS), as well as to exclude underlying disease. Finally, body weight is also very useful in documenting expected weight gain on follow‐up evaluation after treatment of hyperthyroidism; if the expected weight gain does not occur despite cure of the hyperthyroidism, concurrent nonthyroidal disease should be considered.
Despite the advantages of body weight measurement, our study shows that body weight alone fails to accurately reflect body composition or muscle‐to‐fat mass ratio. Furthermore, body weight comparisons across cat populations might fail to reflect body condition differences. Although cats have a more uniform body conformation than either dogs or humans, the ideal body weight of an adult cat can range between 2 and 7 kg, depending on breed, sex, and individual variation.50, 51 Therefore, clinicians should combine body weight with body condition scoring, a widely accepted method that uses both visual observation and palpation of key anatomic features (e.g, fat cover over ribs, abdominal tuck) to assess the amount of body fat. Either a 5‐point or 9‐point BCS system is used to classify cats as too lean, ideal, or too fat.26, 27, 28, 50, 52, 53
However, it is important to realize that BCS classifications are designed to evaluate body fat, but not muscle mass.26, 27, 28 One might expect muscle wasting to only develop in lean cats (low BCS), but loss of muscle mass can also occur in cats with ideal or even high BCS. Our study supports this hypothesis, because only 35% of these cats were thin but over 75% displayed muscle wasting. Therefore, along with evaluation of body fat with BCS, one should also evaluate muscle mass, especially if the cat is losing weight. Clinically, this is performed most easily with a 4‐point muscle scoring system in which muscle mass is accessed at four sites: the skull (temporal muscles), the scapulae, the spine (epaxial muscles), and the wings of the ilia—areas that are relatively devoid of fat and therefore tend to reflect muscle mass rather than fat mass.28, 29, 30, 54
In these hyperthyroid cats, both increasing disease severity and age were associated with a lower body weight, as well as a higher prevalence of low BCS (thinness) and low MCS (muscle wasting). The finding that cats with severe hyperthyroidism weighed less, were thinner, and were more muscle wasted might be predicted, given the known physiological effects of thyroid hormone excess on energy expenditure and muscle and fat loss.2, 4, 6 Studies suggest that older cats (especially those >15 years) tend to lose body weight as they age,55, 56, 57 , 4 which may at least partially explain why these geriatric cats were thinner and had more muscle wasting than the younger hyperthyroid cats. This aging process might also explain why many of these treated cats failed to return to their premorbid weight, recorded one to two years earlier. Even though severity of disease and older age were independently associated with thinness and muscle wasting, the median serum T4 concentration in these geriatric cats was actually lower than T4 concentrations in the younger cat groups, suggesting that age and severity of disease are independent risk factors for fat and muscle loss in hyperthyroid cats.
There are many reasons why senior and geriatric cats might lose weight, including higher daily energy requirements, reduced ability to digest fat and protein, and increased protein turnover.55, 56, 57, 58, 59 , 4 In addition, changes in olfaction or taste or presence of dental disease may lead to a decrease in appetite and subsequent weight loss. Approximately half the cats in our study had dental disease, but it did not appear to decrease their appetite, at least while hyperthyroid. Old cats also tend to lose muscle mass and develop sarcopenia,60, 61, 62 which is also likely related to their decreased ability to digest protein and increased protein turnover, leading to a negative protein balance. Again, hyperthyroidism would be expected to accelerate this age‐related loss of muscle mass caused by the catabolic effects of thyroid hormone excess on muscle tissue.5, 6
Inadequate protein intake by older cats could play a role in pathogenesis of aged‐related sarcopenia. Recently, Laflamme and Hannah63 reported that young adult cats (aged 2–8 years) require >5 g protein/kg body weight per day (≈34% of daily caloric intake as protein) to maintain lean body (muscle) mass. Older senior and geriatric cats appear to require amounts of protein even higher than this to support total body protein turnover and maintain normal muscle mass.64 , 5 In our study, most pet owners fed their cats a combination of both canned and dry foods, generally with multiple flavors or brands of cat food, or both. Therefore, it was impossible to estimate the exact protein intake of these cats; however, as most over‐the‐counter cat foods only contain moderate amounts of protein, it is highly unlikely that most cats ingested a protein intake even close to the recommended amount of >5 g/kg/d. Very few of our owners fed diets known to contain high amounts of protein, as would be needed to fulfill the protein requirements and maintain normal muscle mass, especially in aging cats.
After successful treatment, many of these cats regained both lost fat and muscle mass. However, not all of these treated, euthyroid cats returned to their premorbid weight; some remained thin (about 10%), and almost half remained muscle wasted. In human hyperthyroid patients, lost muscle mass can take several months to recover after successful treatment, so it is possible that additional increases in body weight or muscle mass might occur in these treated cats if studied over a longer time. However, median follow‐up time in the 131 treated cats was >6 months, with no difference in follow‐up time between cats that normalized muscle mass and those that did not. Therefore, failure to completely regain lost weight or restore lost muscle mass is also likely related to aging per se, and not all because of the prior hyperthyroid state alone. In addition, daily dietary protein intake in most of these cats was below that recommended to maintain lean body mass, so relative protein deficiency likely played a role in the failure of some cats to fully recover lost muscle mass. Finally, about 25% of these treated cats developed evidence of chronic kidney disease (CKD) at time of follow‐up, which may have contributed to failure to completely regain weight or restore lost muscle.60
One limitation of our study was our use of subjective, semiquantitative methods to determine both BCS and MCS. Several other more objective techniques can be used to estimate body composition, including carcass composition analysis (not ideal for pet cats), deuterium oxide dilution, bioelectrical impedance analysis, quantitative magnetic resonance, ultrasonography, and dual‐energy X‐ray absorptiometry (DXA)53, 65, 66, 67, 68, 69 Of these, DXA analysis is generally considered as the reference standard for estimating body composition for cats.52, 66, 67, 69 However, the imaging equipment for DXA is not widely available, with its use generally limited to larger research institutions, and we do not have DXA at the Animal Endocrine Clinic. Regardless, DXA analysis measures overall lean body mass, which includes both visceral organs and smooth and skeletal muscle; skeletal muscle tissue accounts for only half of lean body mass,29, 70, 71 making the changes detected on DXA less specific for evaluation of muscle wasting. Nevertheless, additional studies to objectively quantitate muscle mass in hyperthyroid cats are warranted to verify the results of our study.
Another limitation of our study was its possible inherent bias, especially in the cats evaluated before and after the treatment. In our clinic situation, we were not blinded to the fact that the cats had been treated, and therefore, it is possible that this knowledge unconsciously biased our post‐treatment MCSs (making them better than they actually were). We tried to compensate for this potential bias in 2 ways: first of all, we did all of our follow‐up evaluations (body weight, BCS, and MCSs) without prior knowledge of the pretreatment body weight or body and muscle condition scores (i.e, we did not look up their pretreatment data until after our follow‐up examination was completed). Additionally, we compared our follow‐up results of these euthyroid cats to those of 14 cats with persistent hyperthyroidism, evaluated during the same period and at similar intervals as the 117 euthyroid cats, in which the radioiodine treatment failed to induce cure. Based on our results, the results clearly show that the changes in body weight, BCS, and MCS were caused by the cure of the hyperthyroidism, because little to no improvement in any of these parameters occurred in the cats with persistent hyperthyroidism. We do not believe we were biased in these evaluations, as we did not have the results of the serum thyroid function tests for 2–3 days, long after our physical examinations had already been completed.
Finally, other limitations include the questionable reliability of the premorbid weight measurements, which were recorded in hundreds of veterinary clinics on different scales, most of which were likely not calibrated. We also had a wide range of follow‐up times after treatment, ranging from 3 to 12 months. Although we had recommended that cats return for reevaluation between 3 and 6 months, many owners did not return until many weeks or even months later. Because this was a clinical study involving pet cats, we had limited control concerning which cats were reevaluated or the time period of when this evaluation took place.
In conclusion, most hyperthyroid cats evaluated in this study had evidence of weight loss but only a third were thin. However, muscle wasting was very common, suggesting that this contributed to a large proportion of the weight loss commonly reported in cats with hyperthyroidism. After successful treatment, not all cats returned to their premorbid weight, and about half remained muscle wasted. Future studies that use objective methods (e.g, DXA) to quantitate muscle mass in hyperthyroid cats, both before and after the treatment, are warranted to verify the results reported in this study.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Where Work was Performed: Animal Endocrine Clinic, New York, New York, USA. Animal Endocrine Clinic Institutional Animal Ethics Committee approved the study.
Footnotes
Ohaus Digital Scale, Model ES30R Parsippany, NJ
GraphPad Prism, version 6.0; GraphPad Software, La Jolla, CA
MedCalc, version 14.12.0, MedCalc Software, bvnv, Ostend, Belgium
Cupp C, Perez‐Camargo G, Patil A, et al. Long‐term food consumption and body weight changes in a controlled population of geriatric cats (abstract). Compend Contin Educ Vet 2004;26 (2A):60
Laflamme D. Protein requirements of aging cats based on preservation of lean body mass (abstract). In: 13th Annual American Academy of Veterinary Nutrition (AAVN) Clinical Nutrition & Research Symposium, Seattle, Washington 2013;17
References
- 1. Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid 1995;5:481–492. [DOI] [PubMed] [Google Scholar]
- 2. Danforth E Jr, Burger A. The role of thyroid hormones in the control of energy expenditure. Clin Endocrinol Metab 1984;13:581–595. [DOI] [PubMed] [Google Scholar]
- 3. Riis AL, Gravholt CH, Djurhuus CB, et al. Elevated regional lipolysis in hyperthyroidism. J Clin Endocrinol Metab 2002;87:4747–4753. [DOI] [PubMed] [Google Scholar]
- 4. Muller MJ, Seitz HJ. Thyroid hormone action on intermediary metabolism. Part II: Lipid metabolism in hypo‐ and hyperthyroidism. Klin Wochenschr 1984;62:49–55. [DOI] [PubMed] [Google Scholar]
- 5. Riis AL, Jorgensen JO, Ivarsen P, et al. Increased protein turnover and proteolysis is an early and primary feature of short‐term experimental hyperthyroidism in healthy women. J Clin Endocrinol Metab 2008;93:3999–4005. [DOI] [PubMed] [Google Scholar]
- 6. Muller MJ, Seitz HJ. Thyroid hormone action on intermediary metabolism. Part III. Protein metabolism in hyper‐ and hypothyroidism. Klin Wochenschr 1984;62:97–102. [DOI] [PubMed] [Google Scholar]
- 7. Acotto CG, Niepomniszcze H, Mautalen CA. Estimating body fat and lean tissue distribution in hyperthyroidism by dual‐energy X‐ray absorptiometry. J Clin Densitom 2002;5:305–311. [DOI] [PubMed] [Google Scholar]
- 8. Lonn L, Stenlof K, Ottosson M, et al. Body weight and body composition changes after treatment of hyperthyroidism. J Clin Endocrinol Metab 1998;83:4269–4273. [DOI] [PubMed] [Google Scholar]
- 9. Dutta P, Bhansali A, Walia R, et al. Weight homeostasis & its modulators in hyperthyroidism before & after treatment with carbimazole. Indian J Med Res 2012;136:242–248. [PMC free article] [PubMed] [Google Scholar]
- 10. de la Rosa RE, Hennessey JV, Tucci JR. A longitudinal study of changes in body mass index and total body composition after radioiodine treatment for thyrotoxicosis. Thyroid 1997;7:401–405. [DOI] [PubMed] [Google Scholar]
- 11. Da Nobrega AC, Vaisman M, De Araujo CG. Skeletal muscle function and body composition of patients with hyperthyroidism. Med Sci Sports Exerc 1997;29:175–180. [DOI] [PubMed] [Google Scholar]
- 12. Greenlund LJ, Nair KS, Brennan MD. Changes in body composition in women following treatment of overt and subclinical hyperthyroidism. Endocr Pract 2008;14:973–978. [DOI] [PubMed] [Google Scholar]
- 13. Xie LJ, Zhou HJ, Li JF, et al. Redistribution of body composition in patients with Graves' disease after iodine‐131 treatment. Eur J Clin Nutr 2015;69:856–861. [DOI] [PubMed] [Google Scholar]
- 14. Baral R, Peterson ME. Thyroid gland disorders In: Little SE, ed. The Cat: Clinical Medicine and Management. Philadelphia: Elsevier Saunders; 2012:571–592. [Google Scholar]
- 15. Mooney CT, Peterson ME. Feline hyperthyroidism In: Mooney CT, Peterson ME, eds. Manual of Canine and Feline Endocrinology, 4th ed Quedgeley, Gloucester: British Small Animal Veterinary Association; 2012:199–203. [Google Scholar]
- 16. Holzworth J, Theran P, Carpenter JL, et al. Hyperthyroidism in the cat: Ten cases. J Am Vet Med Assoc 1980;176:345–353. [PubMed] [Google Scholar]
- 17. Peterson ME, Kintzer PP, Cavanagh PG, et al. Feline hyperthyroidism: Pretreatment clinical and laboratory evaluation of 131 cases. J Am Vet Med Assoc 1983;183:103–110. [PubMed] [Google Scholar]
- 18. Thoday KL, Mooney CT. Historical, clinical and laboratory features of 126 hyperthyroid cats. Vet Rec 1992;131:257–264. [DOI] [PubMed] [Google Scholar]
- 19. Peterson ME. Subclinical to mild hyperthyroidism in cats: Issues in diagnosis and treatment. In: 2015 ACVIM Forum, Indianapolis, Indiana: 2015. [Google Scholar]
- 20. Eccles M, Grimshaw J, Campbell M, et al. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care 2003;12:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American College of Physicians . A primer on before‐after studies: Evaluating a report of a “successful” intervention. Eff Clin Pract 2002;5:100–101. [PubMed] [Google Scholar]
- 22. Peterson ME. More Than Just T4: Diagnostic testing for hyperthyroidism in cats. J Feline Med Surg 2013;15:765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peterson ME, Guterl JN, Nichols R, et al. Evaluation of serum thyroid‐stimulating hormone concentration as a diagnostic test for hyperthyroidism in cats. J Vet Intern Med 2015;29:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peterson ME, Guterl JN, Rishniw M, et al. Evaluation of quantitative thyroid scintigraphy for diagnosis and staging of disease severity in cats with hyperthyroidism: Comparison of the percent thyroidal uptake of pertechnetate to the thyroid‐to‐salivary ratio and thyroid‐to‐background ratios. Vet Radiol Ultrasound 2016;57:427–440. [DOI] [PubMed] [Google Scholar]
- 25. Peterson ME, Broome MR. Thyroid scintigraphy findings in 2,096 cats with hyperthyroidism. Vet Radiol Ultrasound 2015;56:84–95. [DOI] [PubMed] [Google Scholar]
- 26. WSAVA Nutritional Assessment Guidelines Task Force Members , Freeman L, Becvarova I, Cave N, et al. WSAVA nutritional assessment guidelines. J Small Anim Pract 2011;52:385–396. [DOI] [PubMed] [Google Scholar]
- 27. Laflamme DP. Development and validation of a body condition score system for cats: A clinical tool. Feline Prac 1997;25:13–18. [Google Scholar]
- 28. Baldwin K, Bartges J, Buffington T, et al. AAHA nutritional assessment guidelines for dogs and cats. J Am Anim Hosp Assoc 2010;46:285–296. [DOI] [PubMed] [Google Scholar]
- 29. Michel KE, Anderson W, Cupp C, et al. Correlation of a feline muscle mass score with body composition determined by dual‐energy X‐ray absorptiometry. Br J Nutr 2011;106(Suppl 1):S57–S59. [DOI] [PubMed] [Google Scholar]
- 30. WSAVA Global Nutrition Committee . Muscle condition score. In: 2016: http://www.wsava.org/sites/default/files/Muscle condition score chart‐Cats.pdf (accessed January 12. 2016).
- 31. D'Agostino RB. Tests for normal distribution In: D'Agostino RB, Stephens MA, eds. Goodness‐of‐Fit Techniques. New York: Macel Dekker; 1986:367–420. [Google Scholar]
- 32. Conover WJ. Practical Nonparametric Statistics, 3rd ed New York: John Wiley & Sons; 1999. [Google Scholar]
- 33. Altman DG, Bland JM. Quartiles, quintiles, centiles, and other quantiles. BMJ 1994;309:996–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vogt AH, Rodan I, Brown M, et al. AAFP‐AAHA: Feline life stage guidelines. J Am Anim Hosp Assoc 2010;46:70–85. [DOI] [PubMed] [Google Scholar]
- 35. Pittari J, Rodan I, Beekman G, et al. American Association of Feline Practitioners. Senior care guidelines. J Feline Med Surg 2009;11:763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunn OJ. Multiple contrasts using rank sums. Technometrics 1964;5:241–252. [Google Scholar]
- 37. McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 1947;12:153–157. [DOI] [PubMed] [Google Scholar]
- 38. Salvatore D, Simonides WS, Dentice M, et al. Thyroid hormones and skeletal muscle–new insights and potential implications. Nat Rev Endocrinol 2014;10:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carter WJ, van der Weijden Benjamin WS, Faas FH. Effect of experimental hyperthyroidism on skeletal‐muscle proteolysis. Biochem J 1981;194:685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Angeras U, Hasselgren PO. Protein turnover in different types of skeletal muscle during experimental hyperthyroidism in rats. Acta Endocrinol (Copenh) 1985;109:90–95. [PubMed] [Google Scholar]
- 41. Gelfand RA, Hutchinson‐Williams KA, Bonde AA, et al. Catabolic effects of thyroid hormone excess: The contribution of adrenergic activity to hypermetabolism and protein breakdown. Metabolism 1987;36:562–569. [DOI] [PubMed] [Google Scholar]
- 42. Morrison WL, Gibson JN, Jung RT, et al. Skeletal muscle and whole body protein turnover in thyroid disease. Eur J Clin Invest 1988;18:62–68. [DOI] [PubMed] [Google Scholar]
- 43. Riis AL, Jorgensen JO, Gjedde S, et al. Whole body and forearm substrate metabolism in hyperthyroidism: Evidence of increased basal muscle protein breakdown. Am J Physiol Endocrinol Metab 2005;288:E1067–E1073. [DOI] [PubMed] [Google Scholar]
- 44. Nielsen JM. Thyrotoxic muscular syndromes. Bull Los Angel Neuro Soc 1947;12:184. [PubMed] [Google Scholar]
- 45. Logothetis J. Neurologic and muscular manifestations of hyperthyroidism. Arch Neurol 1961;5:533–544. [DOI] [PubMed] [Google Scholar]
- 46. Ramsay ID. Muscle dysfunction in hyperthyroidism. Lancet 1966;2:931–934. [DOI] [PubMed] [Google Scholar]
- 47. Broussard JD, Peterson ME, Fox PR. Changes in clinical and laboratory findings in cats with hyperthyroidism from 1983 to 1993. J Am Vet Med Assoc 1995;206:302–305. [PubMed] [Google Scholar]
- 48. Caney S. Weight loss in the elderly cat. Appetite is fine and everything looks normal. J Feline Med Surg 2009;11:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Singer P, Attal‐Singer J, Shapiro H. Body mass index and weight change: The sixth vital sign. Isr Med Assoc J 2008;10:523–525. [PubMed] [Google Scholar]
- 50. Lund EM, Armstrong PJ, Kirk CA, et al. Prevalence and risk factors for obesity in adult cats from private US veterinary practices. Intern J Appl Res Vet Med 2005;3:88–96. [Google Scholar]
- 51. Kienzle E, Moik K. A pilot study of the body weight of pure‐bred client‐owned adult cats. Br J Nutr 2011;106(Suppl 1):S113–S115. [DOI] [PubMed] [Google Scholar]
- 52. Bjornvad CR, Nielsen DH, Armstrong PJ, et al. Evaluation of a nine‐point body condition scoring system in physically inactive pet cats. Am J Vet Res 2011;72:433–437. [DOI] [PubMed] [Google Scholar]
- 53. Center SA, Warner KL, Randolph JF, et al. Resting energy expenditure per lean body mass determined by indirect calorimetry and bioelectrical impedance analysis in cats. J Vet Intern Med 2011;25:1341–1350. [DOI] [PubMed] [Google Scholar]
- 54. Baez JL, Michel KE, Sorenmo K, et al. A prospective investigation of the prevalence and prognostic significance of weight loss and changes in body condition in feline cancer patients. J Feline Med Surg 2007;9:411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Harper EJ. Changing perspectives on aging and energy requirements: Aging, body weight and body composition in humans, dogs and cats. J Nutr 1998;128:2627S–2631S. [DOI] [PubMed] [Google Scholar]
- 56. Perez‐Camargo G. Cat nutrition: What is new in the old? Compend Contin Educ Vet 2004;26:5–10. [Google Scholar]
- 57. Laflamme D, Gunn‐Moore D. Nutrition of aging cats. Vet Clin North Am Small Anim Pract 2014;44:761–774. [DOI] [PubMed] [Google Scholar]
- 58. Hutchinson D, Freeman LM. Focus on nutrition–Optimal nutrition for older cats. Compend Contin Educ Vet 2011;33:E1–E3. [PubMed] [Google Scholar]
- 59. Teshima E, Brunetto MA, Vasconcellos RS, et al. Nutrient digestibility, but not mineral absorption, is age‐dependent in cats. J Anim Physiol Anim Nutr (Berl) 2010;94:e251–e258. [DOI] [PubMed] [Google Scholar]
- 60. Freeman LM. Cachexia and sarcopenia: Emerging syndromes of importance in dogs and cats. J Vet Intern Med 2012;26:3–17. [DOI] [PubMed] [Google Scholar]
- 61. Morley JE. Sarcopenia in the elderly. Fam Pract 2012;29(Suppl 1):i44–i48. [DOI] [PubMed] [Google Scholar]
- 62. Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 2014;11:177–180. [PMC free article] [PubMed] [Google Scholar]
- 63. Laflamme DP, Hannah SS. Discrepancy between use of lean body mass or nitrogen balance to determine protein requirements for adult cats. J Feline Med Surg 2013;15:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Laflamme DP. Determining protein requirements: Nitrogen balance versus lean body mass Companion Animal Nutrition Summit: Tackling Myths about Pet Nutrition, Atlanta, Georgia: Nestle Purina; 2013:37–40. [Google Scholar]
- 65. Hendriks WH, Moughan PJ, Tarttelin MF. Body composition of the adult domestic cat (Felis catus). J Anim Physiol Anim Nutr 1997;77:16–23. [Google Scholar]
- 66. Speakman JR, Booles D, Butterwick R. Validation of dual energy X‐ray absorptiometry (DXA) by comparison with chemical analysis of dogs and cats. Int J Obes Relat Metab Disord 2001;25:439–447. [DOI] [PubMed] [Google Scholar]
- 67. Borges NC, Vasconcellos RS, Carciofi AC, et al. DXA, bioelectrical impedance, ultrasonography and biometry for the estimation of fat and lean mass in cats during weight loss. BMC Vet Res 2012;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zanghi BM, Cupp CJ, Pan Y, et al. Noninvasive measurements of body composition and body water via quantitative magnetic resonance, deuterium water, and dual‐energy X‐ray absorptiometry in cats. Am J Vet Res 2013;74:721–732. [DOI] [PubMed] [Google Scholar]
- 69. Lauten SD, Cox NR, Baker GH, et al. Body composition of growing and adult cats as measured by use of dual energy X‐ray absorptiometry. Comp Med 2000;50:175–183. [PubMed] [Google Scholar]
- 70. Davidson LE, Kelley DE, Heshka S, et al. Skeletal muscle and organ masses differ in overweight adults with type 2 diabetes. J Appl Physiol 1985;2014:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Woodard HQ, White DR. The composition of body tissues. Br J Radiol 1986;59:1209–1218. [DOI] [PubMed] [Google Scholar]


