In this issue of Circulation, Parikh and colleagues1 present an elegant study that lies at the intersection of two recent major developments in cardiovascular research–the recognition of the role of microRNAs in normal and abnormal cardiovascular biology, and the introduction of computational systems biology approaches to elucidate the mechanisms of cardiovascular disease. This work provides convincing evidence for the central role of microRNA-21 (miR-21) in the pathogenesis of pulmonary hypertension (PH) generally and pulmonary arterial hypertension (PAH) more specifically, and suggests a potential “two-hit” rationale for incomplete penetrance of mutations in BMPR2 in heritable PAH.
MicroRNAs are small non-coding RNA molecules 17–23 nucleotides long that bind to target messenger RNA (mRNA) molecules and repress their translation by cleavage, sequestration, degradation, or ribosomal inhibition. There are over 1000 microRNAs in the genome, regulating approximately 30% of gene expression.2 Although they were initially discovered in Caenorhabditis elegans in 1993, their role in mammalian cardiovascular biology has been recognized only in the last decade. Global deletion of functional microRNAs in smooth muscle cells (SMCs) in mice leads to impairment of vascular SMC development, differentiation, and contraction, abrogated aortic development, extensive hemorrhage, and embryonic lethality.3 Similar deletion of microRNAs in endothelial cells (ECs) is compatible with life, but leads to impaired angiogenesis.4 Thus, microRNAs are essential for vascular development and function. Further work, some of which is discussed below, has shown that dysregulation of microRNAs is associated with vascular disease.
To establish the role of miR-21 in PH, Parikh and colleagues used an innovative approach integrating network analysis of existing genome-wide mRNA expression data, algorithmic prediction of microRNA targets, previously established concepts of biological pathways involved in PH, and in vitro and in vivo experimentation. First, they established a “consolidated interactome” of molecular interactions known to occur in PH. Second, they identified microRNAs the predicted target lists of which overlapped significantly with this interactome, based on the assumption that the microRNAs that exert the most powerful influence on disease-relevant pathways should regulate multiple members of multiple pathways. Indeed, they found a strong correlation between the number of predicted targets of each microRNA within the PH-network and the number of targets interacting with the PH network. Third, they selected those microRNAs from this subset that were each associated with biological processes already implicated in the pathogenesis of PH, namely, hypoxia, inflammation, and TGF/BMP signaling. This successive refinement process identified 7 microRNAs of interest, of which one was miR-21. They next conducted a series of cell-based and animal experiments showing that miR-21 is upregulated by hypoxia, inflammatory cytokines, and BMPRII activation, that it leads to feedback inhibition of BMPRII signaling, and that it inhibits RhoB and Rho-kinase activity, thereby presumably inhibiting vascular cellular proliferation.
The paradigm introduced by Parikh and colleagues intriguingly raises the possibility of a “two-hit” mechanism to explain incomplete penetrance of mutations in BMPR2 in heritable PAH. Mutations in BMPR2 underlie 69% of heritable PAH and 20% of idiopathic PAH.5, 6 However, a mutation in BMPR2 alone is insufficient to cause PAH. Disease onset varies widely within the same family and among unrelated individuals with the same BMPR2 mutation, ranging from infancy to late adulthood.7 Genotyping of 108 at-risk members of a single family with a BMPR2 mutation demonstrated a penetrance of 50% in females and 40% in males at age 60 years.8 Pulmonary vascular abnormalities have been studied in mouse models with genomic alterations in Bmpr2. In the same heterozygous null knockout mouse model, studies have yielded somewhat discrepant results. Elevated pulmonary arterial pressure and total pulmonary vascular resistance and greater wall thickness of muscularized pulmonary arteries were observed at baseline in one study,9 but only in response to challenges with 5-lipoxygnenase10 and serotonin11 in two others. In a smooth muscle-specific transgenic mouse expressing a dominant negative Bmpr2 under the control of a tetracycline-responsive switch, activation of the mutation caused an increase in pulmonary artery pressure and muscularization.12 The results from the current study suggest that decreased BMPRII signaling in the setting of BMPR2 mutations leads to decreased miR-21 expression. The decreased miR-21 level is insufficient to lead to PAH, but increases susceptibility to other triggers such as hypoxia and inflammation.
Interestingly, while miR-21 appears to be a protective, anti-proliferative agent in the current study, other investigators have uncovered contradictory evidence. For example, in balloon-injured rat carotid arteries, miR-21 promotes SMC proliferation and inhibits apoptosis by downregulation of phosphatase and tensin homolog (PTEN) and upregulation of Bcl-2.13, 14 Furthermore, miR-21 appears to mediate pro-inflammatory responses of vascular ECs under shear stress.15 In idiopathic pulmonary fibrosis, miR-21 appears to be a mediator of fibroblast activation and fibrosis.16 Therefore, miR-21 may exert divergent effects that are specific to different conditions, to different cell types and tissues, and to pulmonary versus systemic vasculature.
Although Parikh and colleagues focused on mir-21, several other microRNAs that they identified but did not study in detail in the current report seem also to play a role in vascular disease. The miR17≈92 cluster appears to modulate angiogenesis in tumors and ischemia17 and STAT3 activation suppresses miR-204 expression, leading to a pro-proliferative and antiapoptotic state in SMCs in PAH.18 While detailed investigation of multiple microRNAs may be beyond the scope of any single manuscript, the putative vascular role of additional microRNA families identified by their approach is an indicator of the robustness of their predictions as well as the complexity of the transcriptional and molecular networks that determine the vascular phenotype in PH.
MicroRNAs are attractive candidates for therapeutic interventions in PH. For example, intratracheal delivery of miR-204 mimics in the MCT rat model of PAH leads to decreased pulmonary pressures, right ventricular hypertrophy, and pulmonary arteriolar remodeling18 and miR-21 antagomirs diminish the severity of experimental bleomycin-induced lung fibrosis in mice.16 Circulating microRNAs may also serve as biomarkers to predict morbidity and mortality, and to guide therapy. Circulating miR-204 levels are negatively correlated with both human and rodent PAH.18 Perhaps more importantly, circulating microRNAs sequestered within exosomes may also play a mechanistic role in disease by mediating cross-talk between organs.19
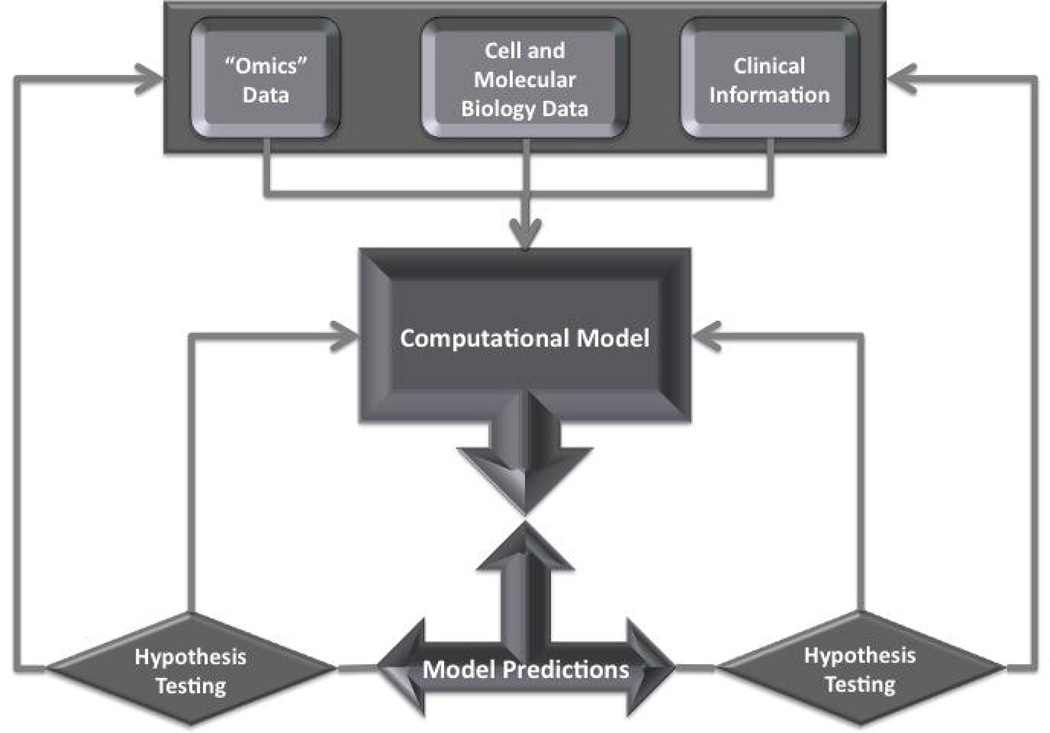
Because microRNAs have impact on multiple genes and pathways, and because disease states are associated with changes in the expression of multiple microRNAs, the development of microRNA-based therapeutic interventions requires an understanding of all the interactions and perturbations that determine the phenotype of an organ—a systems understanding of the organ or the disease. Systems biology is the ambitious new field of biology that integrates computational biology, genomics, proteomics, cell and molecular biology, and physiology, and deals with understanding and modeling of the emergent properties of a system that cannot be predicted from simply cataloging the components of the system. Parikh and colleagues applied systems biology approaches initially to select candidate microRNAs for further study, but subsequently relied on established hypothesis-testing methods to support the biological role of mir-21 in PH. While the importance of these hypothesis-testing experiments cannot be exaggerated, in the end they do not provide us with a real systems level view of PH. To provide such a view, one would need to generate a computational model of PH based on all data available, make a series of predictions with regard to an the effects of perturbations of this model, test the predictions experimentally, refine the model, and retest (Figure 1). Only after several iterations of this process could a working predictive systems level model of PH be defined and used to design and predict response to therapeutic interventions. It is important to note that although the vision of a systems model of PH may seem distant, the manuscript by Parikh and colleagues is an encouraging early step. The exponential growth in the availability of high throughput genomic, epigenomic, transcriptomic, and proteomic data, as well as the development of bioinformatics approaches that allow data integration and mining of published data, facilitate implementation of systems biology models. More importantly, a new generation of cross-disciplinary trainees—computational scientists who are familiar with biological sciences, and biological and medical scientists who are familiar with computational disciplines—is now emerging from a variety of training programs across the country. These scientists have the training to design and complete systems biology experiments, and publications such as the one by Parikh and colleagues should draw their attention and interest to vascular biology and PH. Finally, specifically related to this study, increased accuracy of target prediction algorithms and wider availability of mRNA-microRNA interaction maps in cell cultures and living tissue specimens (such as Ago HITS-CLIP20) will facilitate modeling and refine network predictions.
Figure 1.
Flow of systems biology experiments. The initial model is built based on high throughput prediction, cell and molecular biology data, and clinical information. Model predictions are generated and tested specifically and then fed back into the model to refine it. High-throughput data are also fed back into the database and modify the model through the database channel. This process is repeated iteratively.
In summary, Parikh and colleagues, using systems biology approaches, have identified a central role for miR-21 in the pathophysiology of PAH, and a potential mechanism by which BMPR2 mutations may predispose to, rather than directly cause, PAH. Other microRNAs may also be implicated in PAH, and RhoB may have a heretofore unrecognized role. Their study is an exciting example of the potential of systems biology approaches to transform our understanding of vascular biology in health and disease and should encourage investigators to generate systems level predictive models of PH.
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health (U01HL108642 to FA, HCC, NK; R21 HL109812 to FA; R03 HL095401 to FA; R01HL095397, R01LM009657, RC2HL101715 to NK, FA), the American Thoracic Society (FA), the Pulmonary Hypertension Association (FA), the American Respiratory Alliance of Western Pennsylvania (FA), and The Pittsburgh Foundation (FA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
NK is a co-inventor on a patent application on microRNAs in lung fibrosis and a consultant to Sanofti-Aventis and Stromedix in issues related to lung fibrosis.
References
- 1.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Shaik RS, Waxman AB, Zhang Y-Y, Maron BA, Hartner JC, Fujiwara Y, Orkin SH, Haley KJ, Barabasi A-L, Loscalzo J, Chan SY. A network biology approach reveals that microRNA-21 integrates pathogenic signaling to control pulmonary hypertension. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.060269. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 3.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol. 30(6):1118–1126. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suarez Y, Fernandez-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105(37):14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldred MA, Vijayakrishnan J, James V, Soubrier F, Gomez-Sanchez MA, Martensson G, Galie N, Manes A, Corris P, Simonneau G, Humbert M, Morrell NW, Trembath RC. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum Mutat. 2006;27(2):212–213. doi: 10.1002/humu.9398. [DOI] [PubMed] [Google Scholar]
- 6.Cogan JD, Pauciulo MW, Batchman AP, Prince MA, Robbins IM, Hedges LK, Stanton KC, Wheeler LA, Phillips JA, 3rd, Loyd JE, Nichols WC. High frequency of BMPR2 exonic deletions/duplications in familial pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174(5):590–598. doi: 10.1164/rccm.200602-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA, 3rd, Newman J, Williams D, Galie N, Manes A, McNeil K, Yacoub M, Mikhail G, Rogers P, Corris P, Humbert M, Donnai D, Martensson G, Tranebjaerg L, Loyd JE, Trembath RC, Nichols WC. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet. 2001;68(1):92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, Gruenig E, Janssen B, Koehler R, Seeger W, Eickelberg O, Olschewski H, Elliott CG, Glissmeyer E, Carlquist J, Kim M, Torbicki A, Fijalkowska A, Szewczyk G, Parma J, Abramowicz MJ, Galie N, Morisaki H, Kyotani S, Nakanishi N, Morisaki T, Humbert M, Simonneau G, Sitbon O, Soubrier F, Coulet F, Morrell NW, Trembath RC. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat. 2006;27(2):121–132. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- 9.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1241–L1247. doi: 10.1152/ajplung.00239.2004. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Jones JE, Beppu H, Keaney JF, Jr, Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112(4):553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res. 2006;98(6):818–827. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- 12.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94(8):1109–1114. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
- 13.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100(11):1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 14.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(7200):56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, Li JY, Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 108(25):10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79(4):581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 18.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 208(3):535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosaka N, Ochiya T. Unraveling the Mystery of Cancer by Secretory microRNA: Horizontal microRNA Transfer between Living Cells. Front Genet. 2:97. doi: 10.3389/fgene.2011.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Darnell RB. Mapping in vivo protein-RNA interactions at single-nucleotide resolution from HITS-CLIP data. Nat Biotechnol. 29(7):607–614. doi: 10.1038/nbt.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]