Figure 9.
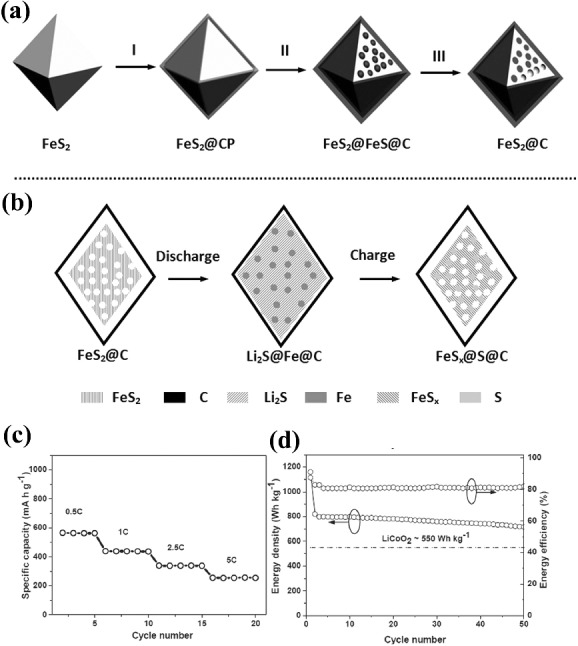
a) Schematic illustration of the fabrication of FeS2@C porous nanooctahedra cathode: I) uniform coating of carbon‐rich polysaccharide (CP) layers onto FeS2 octahedra; II) carbonization of CP layers and partdecomposition of inner encapsulated FeS2; III) removing of acid soluble FeS originated from the decomposed FeS2 by hydrochloric acid. b) Details of the discharge and charge processes of FeS2@C porous nanooctahedra cathode in schematic illustration. c) Rate capability at different rates (increased from 0.5C to 5C); d) discharge energy density and energy efficiency of the FeS2@C porous nanoctahedra vs.cycle number shown along with the theoretical discharge energy density of LiCoO2 cathode (550 Wh kg–1 based on the mass of LiCoO2 only), which is calculated using an average voltage of 3.9 V and a capacity of 140 mAh g–1. Reproduced with permission.148
