Figure 4.
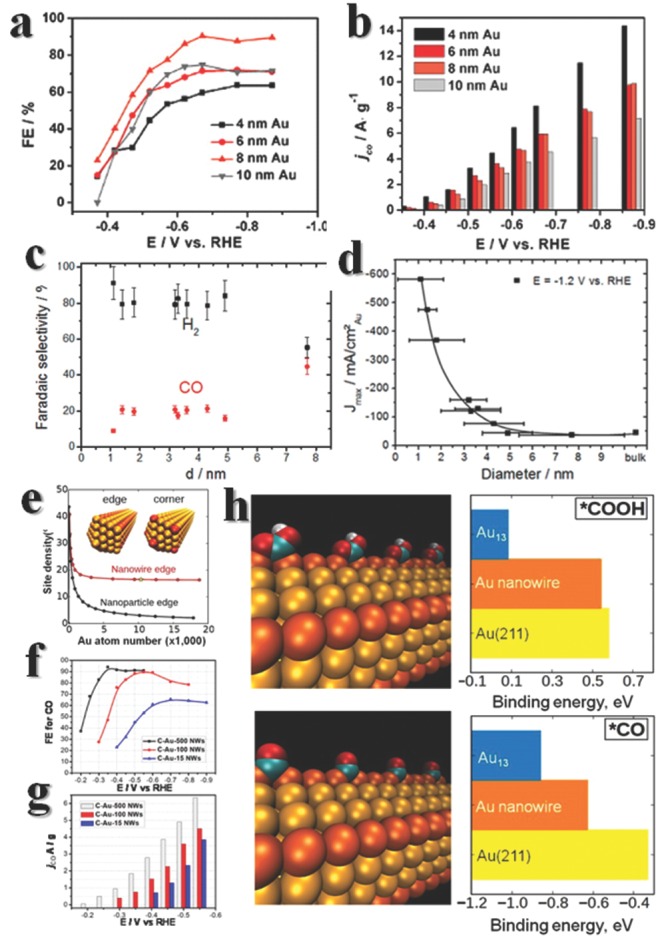
Electrochemical conversion of CO2 results of Au nanomaterials. Here show the performances of gold nanoshperes characterized by a,b) TEM and c,d) STM. Data in (c) were acquired at E = −1.2 V vs RHE. Reproduced with permission.4, 74 Copyright 2013, American Chemical Society. e) While the UNWs get longer, the edge density is much larger than that in nanoparticles. f,g) The property varies with the length of nanowires, the longer the better. C‐Au‐500 (100 or 15) NWs means Au UNWs of 500 (100 or 15) nm length mixed with carbon, respectively. h) Computational results on CO2 reduction and CO formation. Configurations of the adsorbed COOH (up) and CO (down) on a Au NW are portrayed here. The edge‐type sites are highlighted in orange. In the Au‐COOH bonding mode, both C and O (carbonyl) bind to Au directly with OH tilting away from the O=C bond. In the Au‐CO bonding mode, one CO binds to two Au atoms with CO serving as the bridge. Binding energies of the key COOH and CO intermediates calculated on the Au NW, Au13 cluster, and Au(211) are shown in the right. Reproduced with permission.75 Copyright 2013, American Chemical Society.
