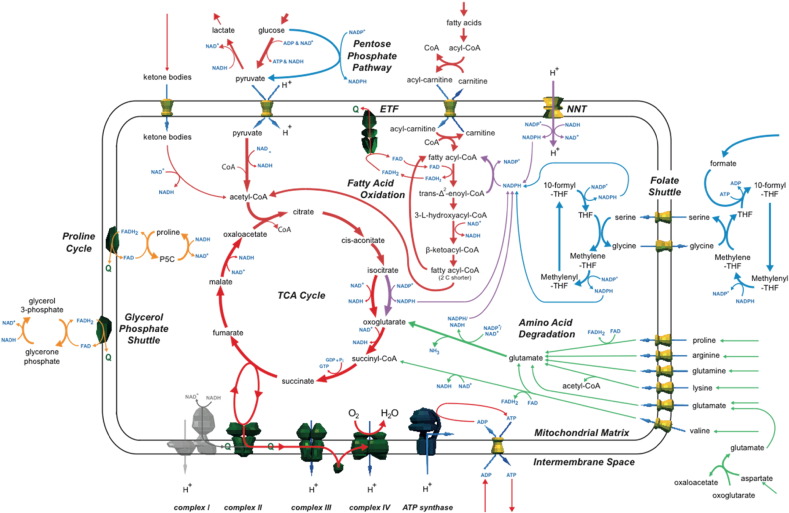
Fig. 2.
Summary of simulations on the effect of complex I deficiency on metabolism.
As flux through complex I was inhibited in the simulations (grey), capacity to oxidise mitochondrial NADH was reduced, affecting the quinone pool, and decreasing ATP production. The model predicted several alternative pathways to compensate for the reduced capacity mitochondrial NADH oxidation and compensate for loss in ATP production. First, the activity of the malate aspartate shuttle was replaced by the glycerol phosphate shuttle, and mitochondrial NADH was oxidised by a proline cycle (orange). Second, glycolysis was reduced (although lactate efflux increased), whereas β-oxidation of fatty acids increased (red). Enzymes for the β-oxidation of fatty acids catalysed a cycle (red and purple) that oxidised mitochondrial NADPH and reduced the quinone pool via FADH2 and the ETF. Third, a hypothetical folate shuttle (blue) emerged that used THF metabolism and the pentose phosphate pathway to import NADPH to the mitochondrion.