FIG. 1.
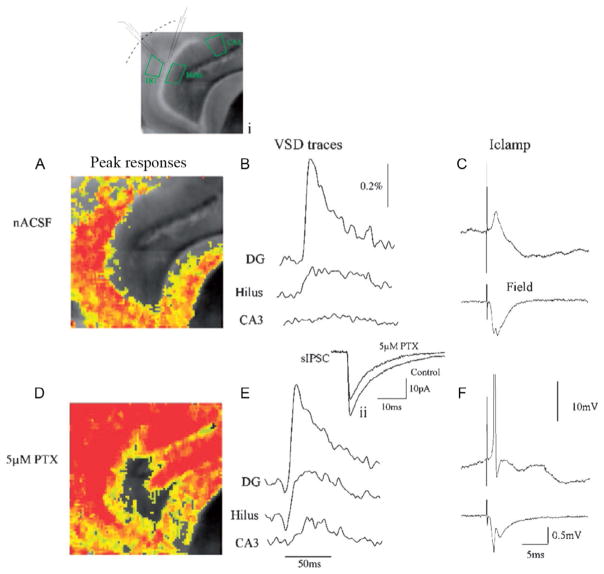
“Gatekeeper” function of the DG is maintained by GABAergic inhibition. Simultaneous voltage-sensitive dye (A), snapshot taken at the peak of the response, (B) trace illustrating the VSD response over time, (C) patch clamp (top) and field potential recording (bottom) of DG response to perforant path activation in control ACSF. (A) Corresponding to a 10–15 mV EPSP in (B), which does not result in activation of downstream structures (note lack of response in area CA3 in (A) and (B)). This lack of CA3 activation is because DGCs do not fire APs in response to perforant path activation under these conditions. This is evident in both the patch (C, top trace, the neuron depolarized to Vm of −50 mV) and field potential recording (C, bottom trace), due to powerful feedforward inhibition activated by perforant path stimulation (C, note large IPSP in patch recording). The importance of inhibition in mediating this “gatekeeper” function is illustrated in responses in (D), (E), and (F), following perfusion with 5 μM picrotoxin, a noncompetitive GABA-A receptor antagonist. This concentration blocks 20–25% of inhibition (see inset [located above (E)] depicting an averaged spontaneous IPSC [sIPSC] before and after perfusion with 5 μM picrotoxin). During 25% GABAergic blockade, perforant path activation resulted in powerful activation of both the DG and downstream structures (CA3 and hilus; D, E). It also triggered AP firing in DGCs (see patch and field potential recordings in (F), both of which exhibit AP firing). A grayscale image of the slice, with patch and field potential recording electrode location is depicted in the inset above (A).
From Coulter, D.A., Carlson, G.C., 2007. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog. Brain Res. 163, 235–243.