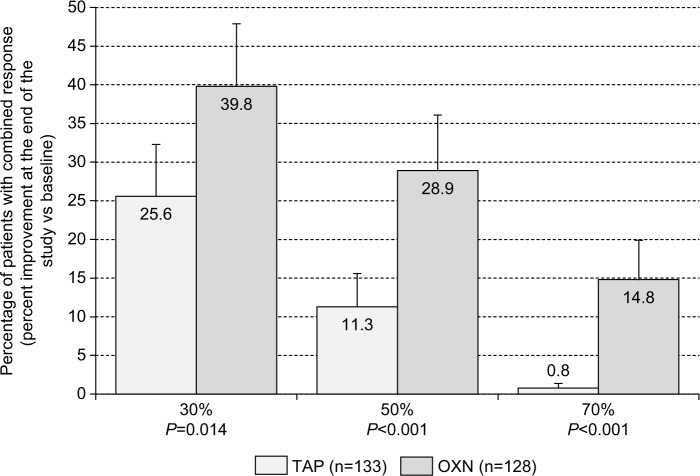
Figure 5.
Proportion of patients (percent ±95% CIs) who recorded a ≥30%/≥50%/≥70% improvement (vs baseline) with respect to all the three efficacy parameters (low back pain intensity index, pain-related disabilities in daily life, and QOL) at the end of the 12-week observation with TAP (n=133, light gray), and OXN (n=128, dark gray) in combination with all the three safety/tolerability parameters as used for the primary end point analysis (eg, no TEAE-related study discontinuation, no CNS side effects, normal BFI at the end of observation).
Abbreviations: 95% CIs, 95% confidence intervals; TAP, tapentadol; OXN, oxycodone/naloxone; QOL, quality of life; CNS, central nervous system; BFI, bowel function index; TEAE, treatment-emergent adverse event.