Figure.
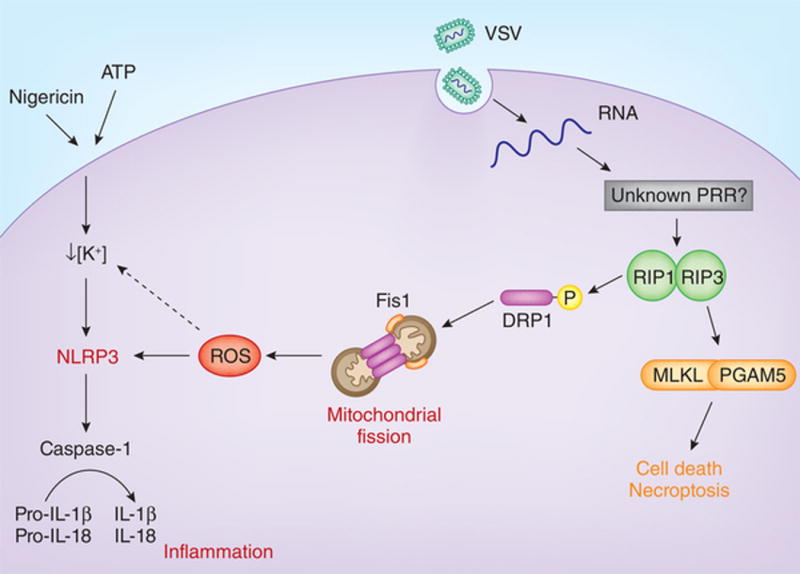
Once activated, NLRP3 leads to the processing of pro-caspase-1 into its active form. Active caspase-1 then cleaves the pro-IL-1β and pro-IL-18 into their secreted, mature forms. Wang et al. show that during infection with RNA viruses such as VSV, the activation of NLRP3 is closely tied to necroptosis. Virus-derived cytosolic RNA induces formation of the RIP1-RIP3 complex, in which RIP1 phosphorylates DRP1. DRP1 then engages Fis1 on the mitochondrial outer membrane, where DRP1 is known to oligomerize to induce mitochondrial fission. Mitochondrial fission leads to increased cytosolic levels of ROS and activated NLRP3. Notably, the authors demonstrate that this arm of RIP1-RIP3 signaling cascade is distinct from the necroptotic arm that is executed by MLKL and PGAM5. Additionally, the mechanisms presented here are also distinct from the activation of NLRP3 by other well-known NLRP3 agonists, such as ATP and nigericin. PRR, pattern-recognition receptor.
