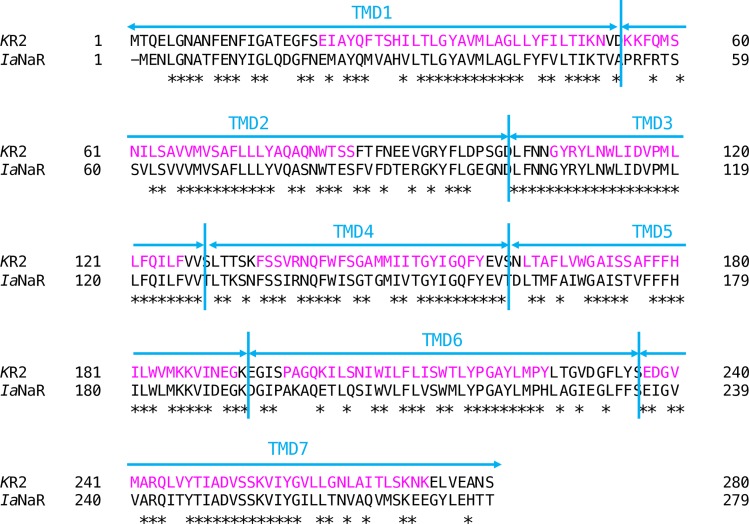
Fig 1. Primary structure of the Na+ pump rhodopsin (NaR) apoproteins.
The sequence alignment of two NaRs, one from Krokinobacter eikastus (KR2, amino acids 1–280) and the other from Indibacter alkaliphilus (IaNaR, amino acids 1–279) is shown. Identical amino acids are indicated by an asterisk. The seven transmembrane helixes of KR2 (Kato et al., 2015) are lettered in magenta. The amino acid sequences of the apoprotein were divided into seven transmembrane domains (TMDs) so that each TMD contain a transmembrane helix (cyan vertical lines). These segments are referred to (from the N-terminal to C-terminal) as “TMD1”, “TMD2”, “TMD3”, “TMD4”, “TMD5”, “TMD6”, and “TMD7”, respectively.