Chronic myelogenous leukemia (CML) is defined by the presence of BCR-ABL1 fusion resulting from t(9;22)(q34;q11.2), its variant translocations, or rarely, cytogenetically cryptic chromosomal rearrangements. BCR-ABL1 is detected as the sole chromosomal abnormality in ~ 90% of CML patients in chronic phase (CP).1,2 With disease progression, however, clonal evolution occurs and additional chromosomal abnormalities (ACAs) emerge. Approximately 60–80% of CML patients in blast phase (BP) have ACAs.3,4
BCR-ABL1 encodes a tyrosine kinase with deregulated activity. Tyrosine kinase inhibitors (TKIs) that target BCR-ABL1 kinase activity have dramatically decreased the progression of CML from CP to BP and improved patient outcome.5,6 The long-term cumulative probability of progression of CML from CP to BP is only about 5%.7,8 Despite the revolutionary progress in the treatment of patients with CML-CP, CML-BP remains a therapeutic challenge. In general, CML-BP is a fatal disease in the era of TKI therapy, with a median survival of only 6–10 months.9 Further investigation into the stratification and optimization of treatment of patients with CML-BP is warranted.
It is believed that the emergence of ACAs in CML is an indication of disease progression and correlates with a poorer prognosis.10,11 However, it is unknown whether ACAs retain the clinical and prognostic impact once the disease progresses to BP in the era of TKI therapy, and if so, whether there is any differential impact in myeloid blast phase (MyBP) vs lymphoid blast phase (LyBP). In this study, we assessed a large cohort of patients with CML-BP diagnosed in the era of TKI therapy, and analyzed the prevalence, treatment response and prognosis of ACAs in patients with MyBP and LyBP.
Cases of CML-BP with t(9;22) or its variant translocations detected by conventional karyotyping analysis from 1999, the 2nd year after TKI therapy was started in our institution, to the present were included in this study. Excluded were cases with BCR-ABL1 detected by fluorescence in situ hybridization or molecular methods but not by conventional karyotyping analysis; cases with blasts showing a mixed phenotype; and cases that presented as isolated myeloid sarcoma or de novo acute leukemia. The time when ACAs emerged was defined as the time of initial diagnosis of CP (CP-ACAs) or the time of the first diagnosis of BP (BP-ACAs) when ACAs were detected. Overall survival (OS) was calculated from the date of diagnosis of BP to the date of last follow-up or death.
A total of 352 patients with CML-BP were included in this study, including 241 (68.5%) patients with MyBP and 111 (31.5%) with LyBP. There were 211 (59.9%) men and 141 (40.1%) women. The median age was 51.6 years at diagnosis of CML-BP (range, 13.2–92.4 years). The median interval from the initial diagnosis of CML to the diagnosis of BP was ~ 23 months (range, 0–232.1 months) in the entire cohort, 25 months in MyBP and 12 months in LyBP patients. The clinical characteristics of all patients are listed in Table 1.
Table 1.
Clinical characteristics of 352 patients with CML, BPa
| Groups | All BP (N= 352) | MyBP (N= 241) | LyBP (N= 111) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Parameter | With ACAs | Without ACAs | P value | With ACAs | Without ACAs | P value | With ACAs | Without ACAs | P value |
| No. (%) | 271 (77) | 81 (23) | 192 (79.7) | 49 (20.3) | 79 (71.2) | 32 (28.8) | |||
| Sex | |||||||||
| Male (N, %) | 171 (63.1) | 40 (49.4) | 118 (61.5) | 19 (38.8) | 53 (67.1) | 21 (65.6) | |||
| Female (N, %) | 100 (36.9) | 41 (50.6) | 0.03 | 74 (38.5) | 30 (61.2) | 0.004 | 26 (32.9) | 11 (34.4) | 0.88 |
| Age (years) | |||||||||
| Median | 52.1 | 48.6 | 52.4 | 55.1 | 50.8 | 46.6 | |||
| Range | 13.2–92.4 | 17.6–90.2 | 15.4–92.4 | 23.3–90.2 | 13.2–77.0 | 17.6–72.9 | |||
| Interval (months) | |||||||||
| Median | 23 | 23.3 | 25.3 | 25 | 11.9 | 11.9 | |||
| Range | 0–232.1 | 0–225.5 | 0–232.1 | 0–193.8 | 0–210.7 | 0–225.5 | |||
| Status at last F/U | |||||||||
| Alive (N, %) | 69 (25.5) | 25 (30.9) | 39 (20.3) | 13 (26.5) | 30 (38.0) | 12 (37.5) | |||
| Dead (N, %) | 202 (74.5) | 56 (69.1) | 0.33 | 153 (79.7) | 36 (73.5) | 0.35 | 49 (62) | 20 (62.5) | 0.96 |
| Tx response | |||||||||
| HR (N, %) | 136/257 (52.9) | 59/79 (74.7) | 0.0006 | 83/180 (46.1) | 32/47 (68.1 ) | 0.007 | 53/77 (68.8) | 27/32 (84.4) | 0.09 |
| CCyR (N, %) | 79/257 (30.7) | 31/79 (39.2) | 0.16 | 40/180 (22.2) | 15/47 (31.9) | 0.17 | 39/77 (50.6) | 16/32 (50) | 0.95 |
| MMR (N, %) | 60/257 (23.4) | 23/78 (29.5) | 0.27 | 29/180 (16.1) | 11/47 (23.4) | 0.24 | 31/77 (40.2) | 12/31 (38.7) | 0.88 |
| Allo-HSCT (N, %) | 70/260 (26.9) | 17/79 (21.5) | 0.34 | 40/182 (22) | 9/48 (18.8) | 0.63 | 30/78 (38.5) | 8/31 (25.8) | 0.21 |
Abbreviations: Age, age at diagnosis of BP; F/U, follow-up; Interval, interval time from the CML diagnosis to the BP diagnosis; Tx, treatment.
Of total 352 patients, 329 were diagnosed with CML initially in CP/AP, and 323 of them (323/329, 98.2%) received TKIs. Of all 352 patients in this cohort, 311 (311/352, 88.4%) received TKIs during BP. Not all patients had treatment response information or status of transplantation available at the last follow-up. Clinical features between different groups were compared using the χ22 test and the Spearman rank correlation. ACA was defined as any additional chromosomal abnormalities in Philadelphia chromosome-positive (Ph+) cells. Chromosomal changes in Philadelphia chromosome-negative cells were not considered as ACAs. BP was defined as 20% or more blasts in peripheral blood or bone marrow. Hematologic response was defined as <5% blasts in bone marrow and 0% in peripheral blood. Complete cytogenetic response was defined as 0% Ph+ metaphases using conventional karyotyping analysis of at least 20 metaphases. Major molecular response was defined as BCR-ABL1:ABL1 transcript ratio ⩽0.1%.
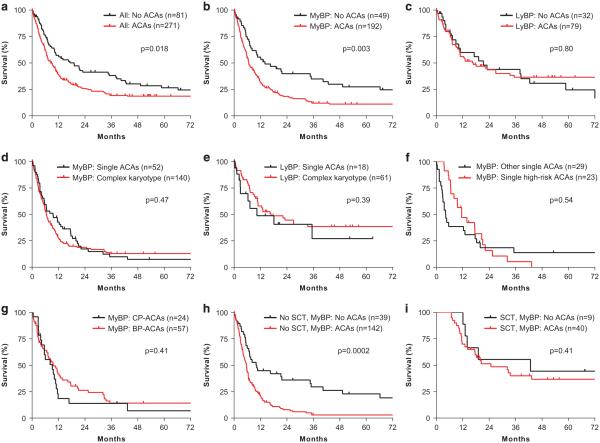
ACAs were detected in 271 (77.0%) patients at time of diagnosis of BP. The frequencies of so-called major-route ACAs in CML-CP were 29.4% for +8, 6.6% for +19, 11.0% for i(17q) and 28.0% for +der(22) of all cases with ACAs in our cohort of CML-BP patients. Overall, CML-BP patients with ACAs had significantly worse OS than those without ACAs (median survival: 8.6 vs 19.1 months, p = 0.018) (Figure 1a). Assessing the MyBP and LyBP patients separately, 192 of 241 (79.7%) MyBP patients and 79 of 111 (71.2%) LyBP patients had ACAs. There was a trend toward a higher ACA ratio in MyBP patients (p = 0.08). As shown in Figure 1b, MyBP patients with ACAs had a significantly worse survival than those without ACAs (median survival: 6.6 vs 14 months, p = 0.003). In contrast, the presence of ACAs had no impact on the OS of LyBP patients (Figure 1c, p = 0.80).
Figure 1.
Differential prognostic impact of ACAs in myeloid vs lymphoid blast phase of CML. (a). Prognostic impact of ACAs in CML, BP (all cases). (b, c) Prognostic impact of ACAs in CML, MyBP (b) and LyBP (c). (d, e) Prognostic impact of the complexity of ACAs in CML, MyBP (d) and LyBP (e). (f) Prognostic impact of single high-risk ACAs vs other single ACAs in CML, MyBP. High-risk ACAs include 3q26.2 rearrangement, i(17q) and − 7/7q del. (g) Prognostic impact of the emerging time of ACAs in CML, MyBP. (h, i) Prognostic impact of ACAs in CML, MyBP, without (h) and with (i) allo-HSCT. Overall survival was calculated from the date of diagnosis of BP to the date of last follow-up or death. A total of 11 patients including 10 with MyBP and one with LyBP were followed-up in outside institutions and their status of transplantation was unknown. Survival curves were built using the Kaplan–Meier method and differences in survival were evaluated by the log-rank test. The study is approved by the Institutional Review Board at the University of Texas MD Anderson Cancer Center.
Given the differential prognostic impact of ACAs on the survival of patients with MyBP vs LyBP, we then examined whether ACAs had any differential clinical impact on hematologic, cytogenetic or molecular response between these two groups (Table 1). MyBP patients with ACAs had a significantly lower rate of hematologic response (HR) than those without ACAs (46.1 vs 68.1%, p = 0.007), whereas LyBP patients with ACAs had a trend toward a lower HR rate (68.8 vs 84.4%, p = 0.09). However, the presence of ACAs had no impact on complete cytogenetic response (CCyR) or major molecular response (MMR) in both MyBP and LyBP patients (Table 1). Accordingly, the presence of ACAs had no impact on the survival of those who achieved CCyR or MMR in both MyBP and LyBP patients (data not shown).
In CML-CP, multiple chromosomal aberrations are associated with poorer prognosis. We investigated whether the complexity of ACAs had prognostic impact. We divided the patients into two groups: one with single ACA and the other with ⩾ 2 ACAs in addition to t(9;22) (that is, complex karyotype). Of 192 MyBP patients with ACAs, 52 had single ACA and 140 had complex karyotypes. Of 79 LyBP patients with ACAs, 18 had single ACAs and 61 had complex karyotypes. In both MyBP and LyBP patients, the complexity of ACAs had no impact on OS (Figures 1d and e).
Since different types of ACAs may have different prognostic impact, we then focused on individual ACAs in CML-BP cases with single isolated ACAs. 3q26.2 rearrangement, i(17q) and − 7/7q deletion are associated with inferior prognosis in CML or/and other myeloid neoplasms and are thus considered as high-risk ACAs.12 When analyzed individually or in combination, however, these ACAs had no impact on the OS in MyBP patients (Figure 1f). When analyzed by major-route (+8, i(17)(q10), +19, +der(22)/ider(22)) vs minor-route single ACAs, no statistical difference in OS was observed between these two groups in MyBP patients (p = 0.60). There were too few cases of LyBP with single ACAs to perform statistical analysis.
We also evaluated the impact of the time when ACAs emerged. This time was available for 81 patients with MyBP and 44 patients with LyBP. For patients with MyBP, 24 had ACAs at diagnosis of CP and 57 had ACAs at diagnosis of BP but not at diagnosis of CP. For patients with LyBP, 14 had ACAs at diagnosis of CP and 29 had ACAs at diagnosis of BP but not at diagnosis of CP. In both MyBP and LyBP patients, the time when ACAs emerged had no impact on OS (Figure 1g and data not shown).
Given the potential curative benefit of allogeneic hematopoietic stem cell transplantation (allo-HSCT),13 we then examined the impact of ACAs in both MyBP and LyBP patients according to the status of allo-HSCT. Without allo-HSCT, MyBP patients with ACAs had markedly poorer survival than those without ACAs (12-month survival: 20.9 vs 44.9%; 24-month survival: 7% vs 36.1%, p = 0.0002) (Figure 1h). In contrast, LyBP patients with and without ACAs had similar survival without allo-HSCT (12-month survival: 30.1 vs 38.9%; 24-month survival: 23.4 vs 26%, p = 0.41). The high fatality of MyBP patients raises the issue of how to manage these patients. As shown in Figure 1i, with allo-HSCT, MyBP patients with and without ACAs had similar but dramatically improved survival (12-month survival: 70 vs 100%; 24-month survival: 51.5 vs 55.6%, p = 0.41). Similarly, LyBP patients with and without ACAs had similar but improved survival (12-month survival: 90.9 vs 100%; 24-month survival: 69.1 vs 75%, p = 0.92). Our data indicates that MyBP patients with ACAs may benefit particularly from allo-HSCT.
The mechanisms responsible for the progression of CML from CP to BP are poorly understood. Persistent expression of BCR-ABL1 promotes genomic instability resulting in chromosomal and molecular changes.14,15 Approximately 80% of patients with MyBP and 70% LyBP in this study cohort acquired ACAs. Patients with MyBP with ACAs had a dismal survival compared with those without ACAs whereas ACAs had no prognostic impact in LyBP patients, suggestive of different genetic basis behind MyBP vs LyBP transformation of CML. Interestingly, although it is well established that complex karyotype, 3q26.2 rearrangement, i(17q) and − 7/7q deletion are poor prognostic factors in CML-CP and other types of myeloid neoplasm, these chromosomal alterations are not prognostically different from any other ACAs in CML-BP. Furthermore, the time the ACAs emerged had no impact on patient survival once the disease reached the stage of BP, consistent with the predominant roles of ACAs in promoting disease progression. Without allo-HSCT, MyBP with ACAs was fatal, with a 24-month survival of only 7%. The dismal outcome of MyBP with ACAs can be significantly improved by allo-HSCT. With allo-HSCT, MyBP and LyBP patients with or without ACAs had similar survival.
In conclusion, ACAs have differential prognostic and clinical impact in CML patients with MyBP vs LyBP. ACAs confer an inferior prognosis in MyBP but not LyBP patients, regardless the complexity of karyotype, the nature and the emerging time of ACAs. MyBP patients with ACAs have 2-year survival of only 7% and those without ACAs 2-year survival of 36% without transplantation. Thus patients with CML in MyBP can be further stratified into two prognostically different subgroups based on the presence or absence of ACAs, and those with ACAs may benefit particularly from transplantation.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
ZC and SH designed the study, collected and analyzed data, and wrote the manuscript. JEC, JLJ, WW, CCY, MJY, EJ, HMK, LJM and SH provided pathology data and clinical information. All authors read and approved the final manuscript.
REFERENCES
- 1.Luatti S, Castagnetti F, Marzocchi G, Baldazzi C, Gugliotta G, Iacobucci I, et al. Additional chromosomal abnormalities in Philadelphia-positive clone: Adverse prognostic influence on frontline imatinib therapy: A GIMEMA Working Party on CML analysis. Blood. 2012;120:761–767. doi: 10.1182/blood-2011-10-384651. [DOI] [PubMed] [Google Scholar]
- 2.Fabarius A, Leitner A, Hochhaus A, Müller MC, Hanfstein B, Haferlach C, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: Long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760–6768. doi: 10.1182/blood-2011-08-373902. [DOI] [PubMed] [Google Scholar]
- 3.Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107:76–94. doi: 10.1159/000046636. [DOI] [PubMed] [Google Scholar]
- 4.Haaß W, Kleiner H, Weiß C, Haferlach C, Schlegelberger B, Müller MC, et al. Clonal evolution and blast crisis correlate with enhanced proteolytic activity of separase in BCR-ABL b3a2 fusion type CML under imatinib therapy. PloS One. 2015;10:e0129648. doi: 10.1371/journal.pone.0129648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambacorti-Passerini C, Piazza R. How I treat newly diagnosed chronic myeloid leukemia in 2015. Am J Hematol. 2015;90:156–161. doi: 10.1002/ajh.23887. [DOI] [PubMed] [Google Scholar]
- 6.Hochhaus A. Educational session: Managing chronic myeloid leukemia as a chronic disease. Hematology Am Soc Hematol Educ Program. 2011;2011:128–135. doi: 10.1182/asheducation-2011.1.128. [DOI] [PubMed] [Google Scholar]
- 7.Kalmanti L, Saussele S, Lauseker M, Müller MC, Dietz CT, Heinrich L, et al. Safety and efficacy of imatinib in CML over a period of 10 years: Data from the randomized CML-study IV. Leukemia. 2015;29:1123–1132. doi: 10.1038/leu.2015.36. [DOI] [PubMed] [Google Scholar]
- 8.Pfirrmann M, Lauseker M, Hoffmann VS, Hasford J. Prognostic scores for patients with chronic myeloid leukemia under particular consideration of competing causes of death. Ann Hematol. 2015;94:S209–S218. doi: 10.1007/s00277-015-2316-0. [DOI] [PubMed] [Google Scholar]
- 9.Saussele S, Silver RT. Management of chronic myeloid leukemia in blast crisis. Ann Hematol. 2015;94:S159–S165. doi: 10.1007/s00277-015-2324-0. [DOI] [PubMed] [Google Scholar]
- 10.Greulich-Bode KM, Heinze B. On the power of additional and complex chromosomal aberrations in CML. Curr Genomics. 2012;13:471–476. doi: 10.2174/138920212802510466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaccaria A, Testoni N, Valenti AM, Luatti S, Tonelli M, Marzocchi G, et al. Chromosome abnormalities additional to the Philadelphia chromosome at the diagnosis of chronic myelogenous leukemia: pathogenetic and prognostic implications. Cancer Genet Cytogenet. 2010;199:76–80. doi: 10.1016/j.cancergencyto.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Cortes JE, Lin P, Beaty MW, Ai D, Amin HM, et al. Clinical and prognostic significance of 3q26.2 and other chromosome 3 abnormalities in CML in the era of tyrosine kinase inhibitors. Blood. 2015;126:1699–1706. doi: 10.1182/blood-2015-05-646489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hehlmann R. How I treat CML blast crisis. Blood. 2012;120:737–747. doi: 10.1182/blood-2012-03-380147. [DOI] [PubMed] [Google Scholar]
- 14.Radich JP. The biology of CML blast crisis. Hematology Am Soc Hematol Educ Program. 2007:384–391. doi: 10.1182/asheducation-2007.1.384. [DOI] [PubMed] [Google Scholar]
- 15.Jabbour EJ, Hughes TP, Cortés JE, Kantarjian HM, Hochhaus A. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia. Leuk Lymphoma. 2014;55:1451–1462. doi: 10.3109/10428194.2013.845883. [DOI] [PMC free article] [PubMed] [Google Scholar]