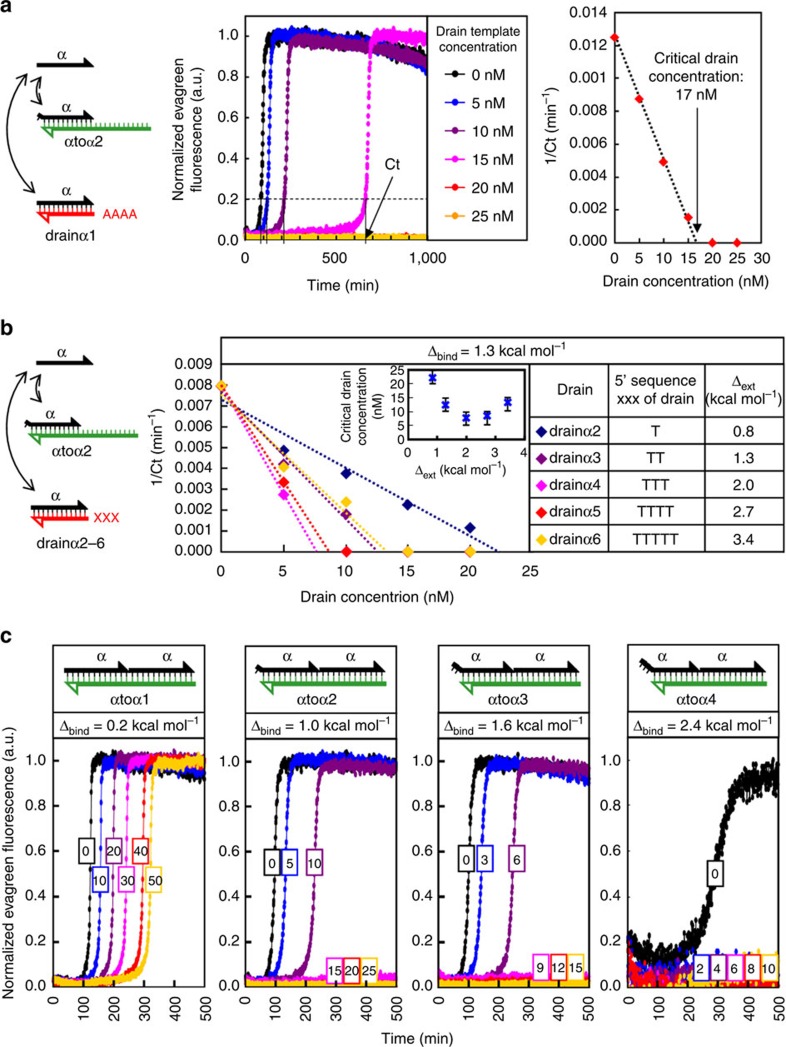
Figure 3. Experimental implementation.
Bst (polymerase), Nb.BsmI (nickase) and ttRecJ (exonuclease) are used to drive an autocatalytic template with or without drains. Fluorescence recordings using an intercalating dye reveal in real time the amplification process. (a) The template αtoα2 was incubated without trigger, in the presence of the indicated concentrations of drain template drainα1 (therefore Δbind=1.0 kcal mol−1 and Δext=2.6 kcal mol−1). For each sample, the amplification delay (Ct, set as the time the fluorescence reaches 20% of its normalized maximum) is extracted. The simple autocatalytic network ([drain]=0) always self-initiates, but increasing concentrations of drain template first delay, then completely abolish this phenomenon. The plot of 1/Ct versus [drain] reveals the hyperbolic transition to bistability and interpolation yields the critical drain concentration. (b) Effect of drain design. To check the influence of Δext on the drain's inhibitory capacity, autocatalytic template αtoα3 was incubated with varying concentrations of various drains (drainα2 to drainα6). These drains all have the same sequence except for the three tailing bases at the 5′-end, therefore Δext values range from 0.8 to 3.4 kcal mol−1. The critical drain concentration is estimated by interpolation and reported in the inset with an error bar corresponding to the highest concentration of drain for which spontaneous amplification is observed and the first bistable point, respectively. (c) Influence of Δbind on the drain's inhibitory capacity. Autocatalytic templates αtoα1, αtoα2, αtoα3 or αtoα4 (schematized in the upper panels) were incubated in the presence of varying concentrations of drainα1 (concentrations indicated in the coloured boxes). Δbind ranges from 0.2 to 2.4 kcal mol−1.