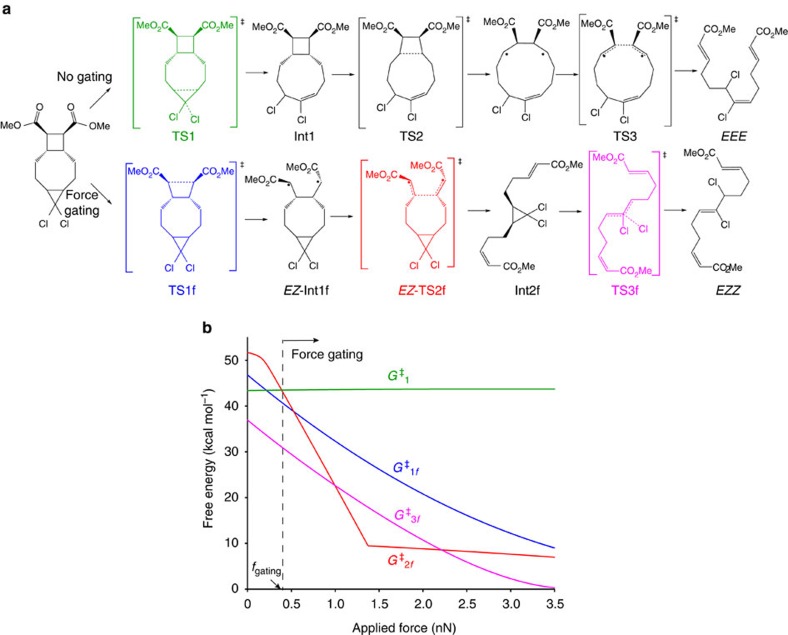
Figure 2. The computed effects of force on the reaction mechanism and activation barriers.
(a) The minimum-energy reaction mechanisms without and with gating are different as are the final products. The relative contributions of the EE and EZ conformers of Int1f and TS2f vary with force (Supplementary Fig. 2): for clarity only one set of isomers is shown. (b) Calculated heights of the free energy barriers (see text for assumptions and details of theoretical methods) in methylbenzoate as a function of the applied force calculated as the differences of the free energies of (i) TS1 and TS1f relative to 5,5-dichlorotricyclo(7.2.0.0)undecane (DCTCU) (G1 ‡ and G1f ‡, respectively), and (ii) TS3f relative to Int2f (G3f ‡); G2f ‡ is the free energy of TS2f relative to (i) DCTCU up to 1.4 nN and (ii) Int1f at >1.4 nN, when Int1f becomes lower in energy than DCTCU. The calculated cross-over between the non-gated and gated reactivity, fgating, occurs at ∼0.45 nN as indicated.